Nuclear medicine firm Digirad of Poway, CA, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Cardius X-ACT imaging system.
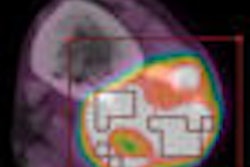
Cardius X-ACT is a cardiac SPECT/volume computed tomography (VCT) imaging system that features a low-dose VCT attenuation correction system that reduces artifacts in images caused by overlying tissues. X-ACT uses a 24-inch-wide detector array and triple-head solid-state digital detectors.
Digirad is working with a number of nuclear cardiology centers, including the Biomedical Institute in Los Angeles, Jefferson Heart Institute in Philadelphia, and University Cardiovascular Medical Group of UCLA in Los Angeles, the company said.
Related Reading
Digirad sells AZ, NV assets, March 6, 2009
Digirad names Slansky as CFO, February 18, 2009
Digirad sales and net loss grow, February 5, 2009
Digirad sells off three service hubs, February 5, 2009
Digirad replaces chief executive, October 23, 2008
Copyright © 2009 AuntMinnie.com