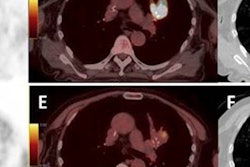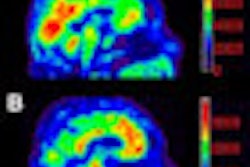
A brain PET scanner based on semiconductor detectors can provide more accurate tumor delineation than a conventional whole-body PET system, according to researchers from Japan, who found that the newly developed system had twice the spatial resolution of conventional PET systems.
Highly conformal radiation treatments, such as intensity-modulated radiotherapy and stereotactic radiosurgery, necessitate precise determination of the target tumor volume. PET with F-18 FDG, widely used for cancer diagnosis and staging, is now also being employed for such target volume delineation. Conventional whole-body PET scanners based on bismuth germanate (BGO) scintillators, however, offer relatively low spatial resolution, making it difficult to determine tumor boundaries on the resulting PET images.
This situation may be improved with the use of a new brain PET scanner based on semiconductor detectors. The scanner -- developed at Hokkaido University in Japan, in collaboration with Hitachi Medical Systems -- is equipped with cadmium telluride (CdTe) semiconductor detectors that directly convert gamma-rays without scintillator material. The CdTe detectors exhibit a spatial resolution of 2.3 mm, compared with 4-7 mm for a whole-body BGO scanner, as well as superior energy resolution (4.1% full-width half-maximum compared with 10% to 20%).
"High-resolution PET with less partial volume effect can offer better identification of active tumor volume than MRI or CT," explained Norio Katoh, assistant professor in the department of radiation medicine at Hokkaido University Graduate School of Medicine. "The CdTe detector has good energy resolution characteristics for use at room temperature. Also, the relatively high atomic number of CdTe means that the CdTe detector has a relatively high stopping power."
 |
| The research team including Norio Katoh (left group, front row, right) and co-authors, radiation oncologists, and Hitachi engineers. |
Previous phantom studies showed that the contrast obtained with the new brain PET scanner was 27% higher than that achieved using the whole-body BGO scanner. Meanwhile, studies of patients with nasopharyngeal carcinoma demonstrated that the brain PET system exhibited more detailed images with sharper tumor edges. The Hokkaido team has now examined the impact of the new scanner on radiotherapy treatment planning (International Journal of Radiation Oncology, Biology, Physics, March 15, 2012, Vol. 82:4, pp. e671-e676).
Clinical comparison
The researchers examined 12 nasopharyngeal carcinoma patients, all of whom underwent FDG-PET scans using both the brain PET scanner and a conventional whole-body PET system. The two scans were performed on the same day, in random order, followed by CT imaging. Gross tumor volumes for the two scan types (GTVNEW and GTVCONV) were visually delineated using just the PET images by an experienced nuclear medicine physician and a radiation oncologist.
The team then used Pinnacle3 to create radiotherapy treatment plans for all of the GTVs, using a prescribed dose to 95% of the planning target volume (PTV, expanded from the GTV) of 2,000 cGy in four fractions. They then calculated dose-volume histograms (DVHs) for the PTV, cerebrum, cerebellum, and brain stem.
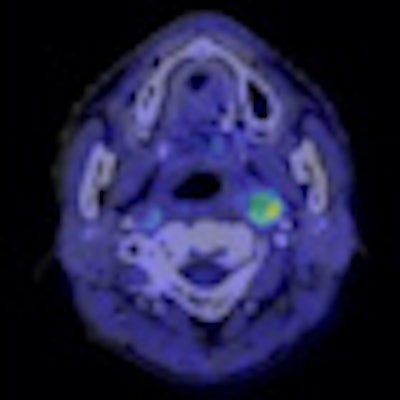
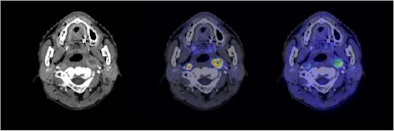 |
| Semiconductor PET images (coregistered with CT images) of nasopharyngeal cancer, using FDG (center image) and F-18 fluoromisonidazole (FMISO) (right image) tracers. The new PET system clearly reveals the different distribution between metabolism and hypoxia in the left neck lymph node metastasis. |
The average absolute volume of GTVNEW was 15.7 mL, compared to 34.0 mL for GTVCONV. Regardless of the order in which the scans were conducted, the GTVNEW volumes were smaller than the GTVCONV volumes for all 12 patients. There were no statistically significant differences between the maximum and mean doses to the PTV for the two regimes.
In the simulated treatment plans, this reduction in target volume resulted in a significant decrease in radiation dose to at-risk organs. In the plan based on GTVNEW, the average maximum doses to the cerebrum/cerebellum and to the brain stem were 2,001 and 1,475 cGy, respectively. For the plan based on the conventional scan, these values were 2,233 and 1,816 cGy.
The authors suggest several causes for the smaller GTV resulting from the semiconductor detector-based PET system. One major factor is that the higher spatial resolution of the brain PET system yielded shaper tumor edges. Other possible reasons include the lower scatter fraction and higher contrast of the new scanner.
The researchers chose not to use CT images when delineating the GTVs, in order to best evaluate the impact of the two PET scanners on treatment planning. They note that the reduction in GTV volumes may be smaller if CT images were used alongside the PET images for GTV delineation. Further studies are also needed to determine the influence on the GTV of the detector geometry and energy resolution, the reconstruction algorithm, and other mechanical factors.
The team is now working to realize clinical use of their scanner. "The next prototype PET system with a wide scan range, up to the lower neck level, is already in operation," said Katoh. "We are now investigating this second machine with semiconductor detectors using a hypoxic tracer, F-18 fluoromisonidazole (FMISO), for radiotherapy planning in patients with head and neck cancers."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.