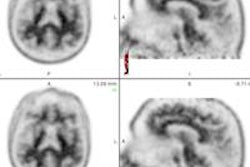
Eli Lilly and Avid Radiopharmaceuticals have received U.S. Food and Drug Administration clearance for Amyvid, a molecular PET imaging agent used for imaging of beta-amyloid plaques in patients with cognitive impairment being evaluated for Alzheimer's disease and other causes.
Amyvid (florbetapir), which binds to amyloid plaques, becomes the first such PET imaging agent approved for evaluation of Alzheimer's disease.
Starting this June, the companies plan to distribute Amyvid to a limited number of radiopharmacies, with the product available in more areas as soon as possible. Because Amyvid loses more than half of its radioactivity every two hours, it must be distributed to radiology departments or imaging centers within several hours of administration to a patient directly from a radiopharmacy, the companies said.
Lilly and Avid caution that the PET images should be interpreted only by radiologists who have successfully completed Amyvid reader training in order to reduce inconsistent interpretation errors related to the estimation of plaque density. Lilly collaborated with the FDA and nuclear medicine experts to develop an online and an in-person reader training program.
Development of the reader-training program was mandated by the FDA in March 2011, when the agency conditionally approved Amyvid with that stipulation, among other requirements.