PET with the radiotracer florbetapir (also known as Amyvid) is an exciting new tool that could help determine which individuals are at risk of developing Alzheimer's disease. But reading the scans can be a challenge, so Pennsylvania researchers enlisted a software application that helped boost reader agreement.
When standardized uptake value ratios (SUVr) were automatically calculated by the software for florbetapir PET images, radiologists were more likely to have similar conclusions. The study's findings could improve consistency in the interpretation of florbetapir PET scans, a known issue with the radiopharmaceutical, according to researchers from the Hospital of the University of Pennsylvania.
Inconsistent interpretation
Florbetapir is a PET imaging agent that specifically binds to beta amyloid, which has been linked to the onset of Alzheimer's disease and other forms of cognitive impairment. It is marketed under the trade name Amyvid in the U.S. and Europe by Eli Lilly and its subsidiary Avid Radiopharmaceuticals.
The U.S. Food and Drug Administration (FDA) in April 2012 approved Amyvid for use in imaging beta-amyloid deposits with PET in adults being assessed for Alzheimer's or other cognitive issues. But the FDA also stipulated that the company develop a reader training program to reduce inconsistent interpretation. Lilly collaborated with the FDA and nuclear medicine experts to develop an online and in-person tutorial program.
Dr. Ameya Nayate, a neuroradiology fellow in the department of radiology and nuclear medicine and lead author of the current study, noted that beta amyloid binds to brain regions such as the frontal lobe, temporal lobe, parietal lobe, and cingulate gyrus. These areas are associated with memory and other cognitive functions.
"With that specific information, we can look at those areas to determine if there is significant beta-amyloid deposition," Nayate said when he presented study results at the 2013 RSNA annual meeting. "However, in order to do that, we need to compare [those brain regions] to an internal standard: an area of the brain that does not have significant beta-amyloid deposition."
One such region is the cerebellum: Prior research has found no significant beta-amyloid deposition in this area, regardless of whether subjects are healthy or have Alzheimer's. SUV could potentially be measured in these regions and compared with SUV in the six brain regions susceptible to beta-amyloid deposits, producing an SUV ratio that indicates whether a patient has a higher burden of amyloid plaque. Physicians could use this information along with clinical findings to estimate a patient's risk of developing Alzheimer's disease.
A previous study of 235 patients found that an increasing SUVr corresponded to greater amounts of beta-amyloid deposition. An SUVr greater than 1.17 indicated an abnormal level of amyloid plaque buildup based on florbetapir uptake, while an SUVr of 1.08 or less meant there was less plaque.
Nayate and colleagues then enlisted the help of five nuclear medicine board-certified readers, who all underwent training via an online tutorial on how to read florbetapir PET brain imaging cases.
The quintet was asked to review 60 cases from the Alzheimer's Disease Neuroimaging Initiative (ADNI), which included patients with no cognitive dysfunction, early mild cognitive impairment, late mild cognitive impairment, and early indications of Alzheimer's disease.
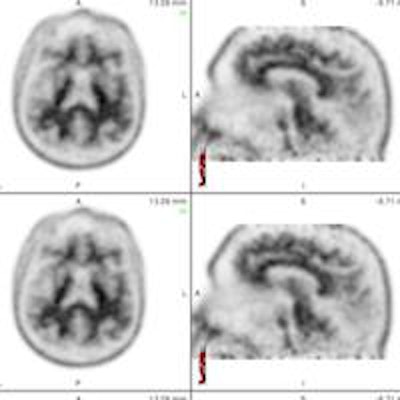
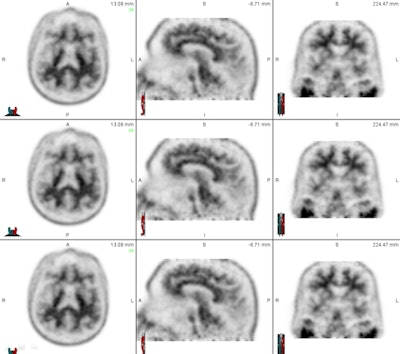 The set of images shows how readers viewed florbetapir PET results from patients in axial, sagittal, and coronal planes to evaluate levels of beta-amyloid deposition. Images courtesy of Dr. Ameya Nayate.
The set of images shows how readers viewed florbetapir PET results from patients in axial, sagittal, and coronal planes to evaluate levels of beta-amyloid deposition. Images courtesy of Dr. Ameya Nayate.The first 30 cases were used as a training exercise because not all five nuclear medicine readers were familiar with florbetapir PET imaging. Each of the 30 cases was read with SUVr information generated by image processing software (syngo Scenium, Siemens Healthcare) and then with no SUVr information.
The readers were asked to determine if there was significant beta-amyloid deposition based on their visual analysis (no SUVr data) and then with the additional SUVr information generated by Scenium. Cases were randomly assigned to four reading sessions, with 15 cases interpreted per session. No case was repeated within an individual session.
"None of the readers were told how the other readers read the studies, and the 1.17 SUVr was used as a reference point and not as an 'end all, be all,' " Nayate explained. "Even if the [SUV] was above or below [1.17], the reader still had to use their overall judgment to determine if there was significant beta-amyloid deposition based on the imaging."
After that training task was completed, 29 of the other 30 cases were evaluated using the same process. (One of the 30 studies was excluded due to misregistration.) Again, each study was interpreted with Scenium-generated SUVr and with no SUVr data.
Interreader agreement
The researchers used the kappa (κ) coefficient to measure interreader agreement, with a scale of 0 to 1 with increments of 0.2. The categories were as follows: 0-0.2 for slight agreement, 0.2-0.4 for fair agreement, 0.4-0.6 for moderate agreement, 0.6-0.8 for high agreement, and 0.8-1 for near-perfect agreement.
When the florbetapir PET studies were read qualitatively with no SUVr information, there was agreement in 21 (72%) of 29 studies (κ = 0.71). When the same PET studies were read with SUVr, there was agreement in 28 (96%) of the 29 studies (κ = 0.94).
"Use of SUVr to quantify F-18 florbetapir uptake in the brain significantly improved interrater agreement to near perfect," Nayate and colleagues concluded.
"This is important because this could potentially be used in the clinical setting if a patient gets a scan at one hospital and gets a second scan at another hospital," Nayate said. "You want to make sure that the same study is read in a similar manner" and have physicians agree on the level of beta-amyloid deposition in a patient's brain.
Further study is needed to confirm the software's effect on the accuracy of the florbetapir PET interpretations, he added. Nayate received RSNA's Trainee Research Prize - Resident for this research.
Study disclosures
Nayate received a grant from Siemens Healthcare.