
Start-up firm Brain Biosciences is hoping to ride the wave of interest in PET scanning for Alzheimer's and other brain diseases by commercializing CerePET, a portable PET scanner designed to offer more cost-effective neuroimaging than whole-body scanners.
Brain Biosciences was first buoyed by a $2.1 million grant from the U.S. National Institute of Mental Health (NIMH) soon after the company's inception in early 2013.
In the words of co-founder David Beylin, CerePET went from "zero to a working system in about eight months." CerePET is still an investigational device and not yet cleared for clinical use by the U.S. Food and Drug Administration (FDA).
The rapid development of CerePET is due in part to its creators' experience in PET technology. Beylin was an early employee of PET developer Naviscan. He and his Brain Biosciences colleagues served as the technical team that developed Naviscan's positron emission mammography (PEM) scanner.
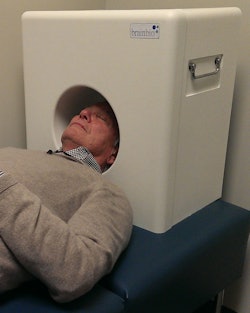 The CerePET prototype weighs approximately 50 lb, so it can be carried from room to room as needed. Image courtesy of Brain Biosciences.
The CerePET prototype weighs approximately 50 lb, so it can be carried from room to room as needed. Image courtesy of Brain Biosciences.
"We learned a lot about making a very small, totally mobile PET system," Beylin said.
After investors moved Naviscan to the West Coast, Beylin and his colleagues chose to stay on the East Coast and create Brain Biosciences.
"As the neurological PET imaging market began to develop, we decided to get together and just go for it," he said.
CerePET technology
During a presentation at the RSNA 2013 conference, Beylin noted that current PET scanner technology relies on large whole-body systems designed for oncology.
PET instrumentation is "increasingly complex and expensive," he said in Chicago, and the use of whole-body PET for amyloid imaging, which is being used to investigate Alzheimer's disease and dementia, is "not economically sensible."
"Less-expensive, high-performance PET devices optimized for neuroimaging are needed," he said.
CerePET's design is fairly straightforward. The device features crystals made from lutetium yttrium orthosilicate (LYSO) technology, a bore diameter of 25 cm, and a 20-cm axial field-of-view.
The scanner has a head-support system that fits on a standard examination bed and is designed so that the scanner gantry does not obstruct the patient's line of sight. The configuration helps patients with claustrophobia and those with neurological or psychiatric conditions who feel the need to be in visual contact with a caregiver or healthcare staff member at all times, Beylin said.
Preclinical work with phantoms showed that the system is capable of spatial resolution of 2 mm to 3 mm across the field-of-view, energy resolution of less than 13% for all detector blocks, and more than 15% image uniformity. Quantitative accuracy was also better than 10% after calculating attenuation correction.
Commercialization plans
Brain Biosciences plans to target radiologists for the system, given that there are more than 8,000 MRI centers accredited by the American College of Radiology (ACR) and even more facilities with access to a CT scanner that have no access to PET.
"Brain MRI is standard workup for dementia, so in that sense [imaging centers] should be offering PET scanning services as well," Beylin said. "We think we can deliver these systems economically. Given that it is a mobile system, one system could be used in multiple offices."
Brain Biosciences has set the groundwork to bring CerePET to human trials by midyear. The company is also interested in collaborating with others developing neuroimaging PET agents and offering the technology for multicenter clinical trials.
"We think [CerePET] will be of great interest to neuro researchers because now they have to compete with oncologists to get access to a PET scanner," he added.
Amyloid imaging
PET imaging for dementia began with the Pittsburgh Compound B (PiB) radiopharmaceutical, which highlighted beta-amyloid deposits in regions of the brain associated with neurological diseases such as Alzheimer's.
Things took a step forward in April 2012, when Eli Lilly and Avid Radiopharmaceuticals received FDA clearance for Amyvid (florbetapir), which binds to amyloid plaques. In October 2013, the FDA cleared GE Healthcare's Vizamyl (flutemetamol) for use with PET imaging of the brain in adults being evaluated for Alzheimer's and dementia.
Several other amyloid imaging agents are currently being developed, including F-18 florbetaben by Piramal Imaging.
"With the advent of amyloid imaging, it is becoming clear that new neuroimaging agents will be approved, and that could create a good commercial trajectory for the company," Beylin said. "You have to be patient with new technology like amyloid imaging, because it will take time for reimbursement to happen, and it will take time for the medical community to start using it. But this is a very useful tool."
As part of its NIMH grant, the company is scheduled to begin a clinical study before the end of this year at Banner Alzheimer's Institute in Phoenix.




















