Contrast agent developer Lantheus Medical Imaging plans to proceed with a phase III program for its flurpiridaz F-18 PET radiopharmaceutical, based on an interim analysis of data from its first phase III study.
To date, approximately 900 subjects have been imaged with flurpiridaz F-18, according to Lantheus. The phase III program includes two trials with 1,400 patients at clinical sites in North America and Europe. Both phase III clinical studies have received a special protocol assessment from the U.S. Food and Drug Administration (FDA), and the first has passed the interim analysis assessment milestone.
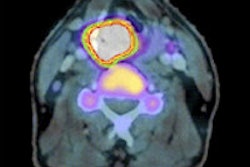
The primary objective of the phase III program is to assess myocardial perfusion using PET with flurpiridaz F-18 in patients with known or suspected coronary artery disease, Lantheus said.