A majority of researchers in a major Alzheimer's disease clinical study believe that study participants should be given their results, if guidance and counseling are also provided, according to results published online August 21 in Neurology.
The survey included 139 clinical trial leaders and coordinators from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Of the respondents, 73% supported disclosing amyloid imaging results to study participants with mild cognitive impairment, and 58% advocated giving amyloid imaging results to those with normal cognition.
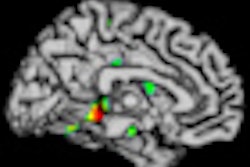
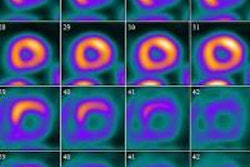
Currently, ADNI has a policy not to disclose results to participants, but the survey showed a growing trend of experts who would favor revising this policy. In addition to finding amyloid imaging results valuable, Alzheimer's experts also valued other biomarker data collected in ADNI, such as spinal fluid tests, PET imaging, and other psychometric tests, suggesting that if amyloid imaging results were allowed to be disclosed, it would likely lead to disclosure of other test results.
While the poll is not a call to immediately disclose biomarker results to subjects, the opinions show that researchers increasingly want to share valid and meaningful results with participants, said co-senior author Dr. Jason Karlawish, professor of medicine and medical ethics and health policy at the University of Pennsylvania, in a statement.