CHICAGO - Siemens Healthcare is launching a range of new products at this week's RSNA meeting, including the third generation of its dual-source CT architecture, a new angiography system, and a major ultrasound technology upgrade.
CT
Somatom Force represents the third iteration of Siemens' dual-source CT design, which uses two sets of x-ray tubes and detector arrays, and is the new flagship product in the Siemens product line. The system is designed for even faster scanning than earlier generations of dual-source systems, with resulting improvements in radiation and contrast dose, as well as better image quality.
Force sports an acquisition speed of 737 mm per second, which means an entire chest study can be performed in one second -- without requiring patients to hold their breath. The rapid acquisition speed also has benefits in cardiac imaging, with the scanner able to produce diagnostic-quality images at low radiation dose for patients at heart rates up to 90 beats per minute, without the use of beta-blockers.
 Siemens is showcasing its new Somatom Force CT scanner at RSNA 2013.
Siemens is showcasing its new Somatom Force CT scanner at RSNA 2013.Siemens is also discussing the benefits of the scanner's design for lung cancer imaging. Built into the design of Force are spectral filters that Siemens calls selective photon shields, which optimize the x-ray spectrum to improve contrast between air and soft tissue. Siemens said that early research indicates Force can acquire lung scans at doses as low as 0.1 mSv.
With respect to contrast use, Force is able to scan at lower kV values, which means that less contrast is required, exposing patients to less iodine. For a typical transcatheter valve implantation (TAVI) runoff study, an exam with Force would require 20 to 30 mL of contrast, compared with 100 mL, previously.
Somatom Force is pending 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is being shown as a work-in-progress.
Another new CT development in the Siemens booth is new configurations of the Somatom Perspective scanner, which now comes in a 16-slice version in addition to the 32-, 64-, and 128-slice options that were available. Siemens is touting the scanner's e-mode for scanning with less wear and tear on the system, as well as its dose reduction features.
Interventional x-ray
Siemens is also touting Artis one, a new interventional x-ray system that targets hospitals that either don't have access to interventional imaging or are considering adding a third or fourth system. The unit was designed to perform standard procedures efficiently, without the specialized functions that might be available in a first or second angiography room.
The system features a small footprint that enables it to be sited in rooms as small as 25 sq meters, versus 45 sq meters for most angiography systems. It also uses up to 20% less power than the previous generation of Artis, and the unit can be installed in 3.5 days, compared with a week, previously. The unit also has a larger 30-inch display.
 Siemens' new Artis one interventional x-ray system.
Siemens' new Artis one interventional x-ray system.For Artis one's imaging chain, Siemens used the same technology found on high-end systems such as the Artis zee, including the detector, Megalix CAT Plus x-ray tube, and image processing chain.
Artis one also sports a new approach to table peristepping, a technique used when following a bolus injection down the periphery of a patient to see if there is a blockage in the legs. With most interventional x-ray systems, the table steps and the angiography unit remains stationary.
Artis one will be offered with a floating tabletop, but it will not be available in a hybrid operating room (OR) configuration -- a Siemens customer would buy an Artis zee or Artis Q system for that purpose. It might not be the best choice for a dedicated electrophysiology (EP) lab.
Siemens is in the process of filing a 510(k) application for Artis one. The company expects to be shipping internationally in the first half of 2014.
Ultrasound
In ultrasound news, Siemens is highlighting its new HELX Evolution upgrade from the Acuson S portfolio of ultrasound scanners. HELX Evolution is powered by the company's new SieStream HD system architecture, designed for high-definition image quality, enhanced workflow, and cost-effectiveness.
Existing ultrasound systems can be upgraded to HELX Evolution, which features a 21.5-inch LCD monitor. The device also increases image visibility in PACS by 20%. The package includes two new transducers, enhanced image processing, and the ability to add new technologies as they become available.
HELX Evolution is 510(k)-cleared by the FDA. Shipping began in late November.
Siemens is also touting several HELX Evolution features: Virtual Touch imaging (VTi), Virtual Touch quantification (VTq), Virtual Touch IQ (VTIQ), and eSie Touch elasticity imaging, designed to visualize and measure tissue stiffness so clinicians can better evaluate anatomy.
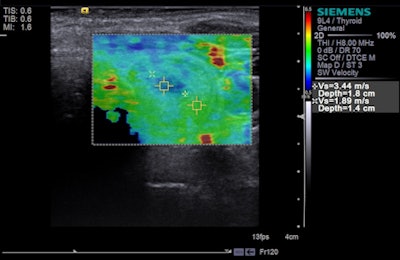 Tissue strain analysis of a thyroid lesion using Virtual Touch IQ. Image courtesy of Siemens.
Tissue strain analysis of a thyroid lesion using Virtual Touch IQ. Image courtesy of Siemens.VTIQ is based on Siemens' acoustic radiation force impulse (ARFI) technology, and physicians can quantifiably assess the stiffness of tissue in small parts, such as the breast and thyroid. Numerical values are shown simultaneously against a qualitative color map over the user-defined region of interest for greater detail of abnormalities within tissue.
Previously available exclusively on the Acuson S3000 system, VTIQ is now offered on the Acuson S2000 HELX Evolution model as well.
VTi and VTIQ are currently available, whereas VTq is pending FDA clearance.
MRI
New enhancements are the order of the day in the MRI section of Siemens' booth. Two new imaging sequences are being introduced as the FREEZEit package for the company's Magnetom Skyra 3-tesla and Magnetom Aera 1.5-tesla systems. The sequences are intended to make MRI available for a broader range of patients.
StarVIBE is a new pulse sequence that enables free-breathing, contrast-enhanced liver imaging for patients who are unable to easily manage breath-holding, such as those who are old, very ill, or young. Meanwhile, TWIST-VIBE is intended for more accurate contrast imaging in dynamic liver MRI scans for all patients and lesions, allowing faster liver imaging with full 4D coverage.
Another new MRI introduction at this year's meeting is Quiet Suite, a new set of applications that enable neuro and musculoskeletal exams with a 70% reduction in sound pressure at the same level of image quality. Quiet Suite will also be available on the Skyra and Aera scanners, with other Siemens magnets to follow; the technology has 510(k) clearance.
In other MRI developments, Siemens is showing a 24-channel version of the Aera scanner as a more affordable option than the 48- and 64-channel options now available.
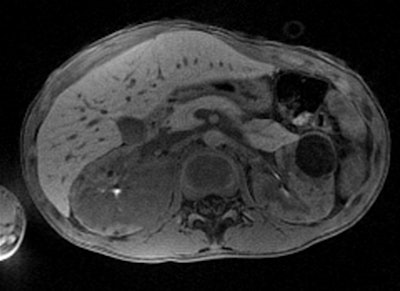 With Siemens' StarVIBE technology, no breath-hold is needed. The images above and below show the increase in image quality with StarVIBE. Images courtesy of Siemens.
With Siemens' StarVIBE technology, no breath-hold is needed. The images above and below show the increase in image quality with StarVIBE. Images courtesy of Siemens.
Advanced visualization
In advanced visualization developments, Siemens is pointing to version VA30 of its syngo.via 3D routine and advanced reading software.
The latest release features the addition of "anatomical intelligence" capabilities for diagnostic reading, as well as new applications and functionalities aimed at further streamlining and accelerating the software, according to the vendor. For example, syngo.via will now include an automatic rib-labeling feature that identifies and labels ribs in CT scans. VA30 also includes a broad range of new applications such as CT stent planning, liver analysis, and body perfusion.
In addition, VA30 includes the General Engine software package, which offers an anatomical range presets feature. Based on Siemens' automatic landmarking and parsing of human anatomy (ALPHA) technology, anatomical range presets automatically recognize anatomy such as shoulders, spines, hips, etc., in clinical images and optimize how they are displayed, according to the vendor.
The second main component of the General Engine package is an advanced reporting tool designed to assist radiologists in creating more quantitative reports by automatically transferring measurements into a structured report. Findings from multiple examinations can also be consolidated into a single report, which makes it easier for doctors to fully assess a patient's condition, Siemens said.
 Siemens is highlighting its syngo.via software.
Siemens is highlighting its syngo.via software.Some aspects of syngo.via VA30 are pending FDA 510(k) clearance, and general availability of VA30 is expected in the spring of 2014.
Molecular imaging
In molecular imaging, Siemens is showing its new Biograph mCT Flow PET/CT system, which is designed, in part, to overcome limitations of conventional bed-based PET/CT. The FlowMotion technique allows patients to be moved through the system's gantry more smoothly, while PET images continue to be acquired.
Biograph mCT Flow with FlowMotion also enables imaging protocols to be based on a particular organ and region of interest in order to enhance resolution. The technique supports accurate, reproducible quantification in all dimensions for precise disease characterization in therapy monitoring, while lowering radiation dose, Siemens said. Additionally, the combination of the scanner's 78-cm bore with five-minute ultrafast scanning and a continuous sense of progress throughout the scan offers a more comfortable exam experience for the patient.
Siemens also brought to RSNA its Symbia Intevo, the first system within its xSPECT product portfolio. Symbia Intevo integrates data from both SPECT and CT modalities to generate high-resolution and quantitative SPECT images.
The company's new xSPECT modality reconstructs both the SPECT and CT portions of the image using the high-definition CT frame of reference. This allows precise alignment that facilitates the extraction and integration of clinically relevant information.
Biograph mCT Flow and Symbia Intevo have both received FDA clearance.
Women's imaging
In women's imaging, Siemens is discussing its Mammomat Inspiration Prime Edition mammography system, which is designed to reduce radiation dose to patients by as much as 30% with no adverse effect on image quality. The device features a new algorithm for progressive image reconstruction, which lowers radiation dose while maintaining image quality by replacing the standard scatter radiation grid.
By identifying scatter-causing structures and correcting the image, the new algorithm allows the complete use of primary radiation so physicians can achieve high-quality images with less dose, Siemens said.
Mammomat Inspiration Prime Edition has received 510(k) clearance from FDA and shipments are underway.
X-ray
The highlight in the radiography section of Siemens' booth is the new Multiple Advances in X-ray (MAX) line of digital radiography (DR) detectors. The new line comes in both static and dynamic versions for classic radiography and fluoroscopy, respectively.
The MAX line comes in four models: MAX Wi-D is a 14 x 17-inch wireless panel; MAX mini is a 10 x 12-inch wireless detector for small field-of-view imaging of patients such as neonates; MAX static is a 17 x 17-inch detector for classic radiography; and MAX dynamic is a 17 x 17-inch detector for fluoroscopy.
Siemens is also showing a new version of its Ysio system, Ysio MAX, which uses the new detectors. The system is pending 510(k) clearance. Another unit, Luminos Agile MAX, has also been upgraded to MAX technology for radiography/fluoroscopy imaging.