Blue Earth Diagnostics has opened a U.S. office in Massachusetts for sales, marketing, and medical affairs support for the development and commercialization of its PET radiopharmaceutical fluciclovine.
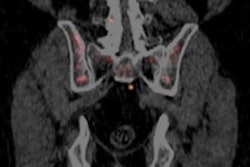
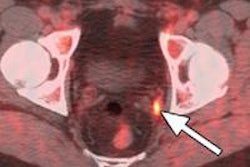
The move comes five months after the U.S. Food and Drug Administration (FDA) gave priority review for the London-based company's new drug application (NDA) filing for fluciclovine, which is intended for lesion detection and localization for prostate cancer patients experiencing biochemical recurrence.
Blue Earth Diagnostics CEO Jonathan Allis, PhD, said the expansion into the U.S. is a "critical next step in Blue Earth's mission to deliver new molecular imaging agents to cancer patients with unmet medical needs."
The company also announced several appointments to its U.S. office:
- Michael Heslop was named president of Blue Earth Diagnostics. He most recently served as vice president of business development and strategy at Lantheus Medical Imaging.
- Peter Gardiner was named vice president of medical affairs for the U.S. His career includes service at G.D. Searle, Gensia, DuPont Pharmaceuticals, Bristol-Myers Squibb, BG Medicine, and Navidea Biopharmaceuticals.
- Bradley Pounds has become vice president of sales for the U.S. He previously served in marketing, medical affairs, and sales positions for Lantheus, Lilly USA, and Navidea.
- David Pendleton was named vice president of marketing. His has held marketing, sales, and business development positions at New England Nuclear, DuPont Medical Imaging, Bristol-Myers Squibb, Theseus Imaging, inVentiv Health, Lantheus, and Navidea.
- Christa Dhimo has become vice president of strategy and execution for the U.S. She most recently was the senior director of strategic programs at Lantheus.
- Priscilla Harlan is the company's vice president of corporate communications. Her career includes executive positions at Lantheus, Molecular Insight Pharmaceuticals, and Sepracor (now Sunovion).