The U.S. Food and Drug Administration (FDA) has approved a new PET radiopharmaceutical from Blue Earth Diagnostics for detecting recurrent prostate cancer.
Called Axumin, the agent is intended for PET imaging of men who are suspected of having recurrent prostate cancer, based on elevated prostate-specific antigen (PSA) levels that occur after primary treatment. Axumin contains a fluciclovine amino acid labeled with an F-18 radioisotope.
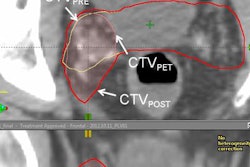
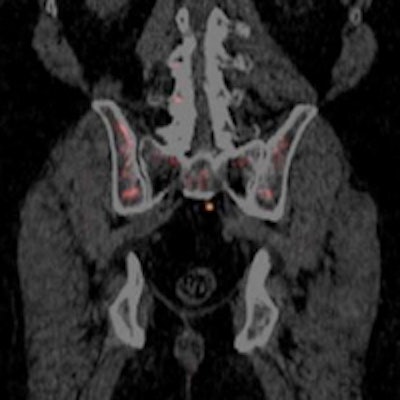
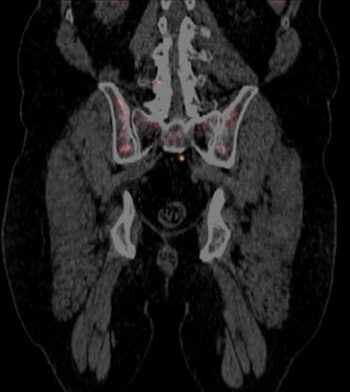 PET scan with Blue Earth's Axumin radiopharmaceutical. Image courtesy of Blue Earth Diagnostics.
PET scan with Blue Earth's Axumin radiopharmaceutical. Image courtesy of Blue Earth Diagnostics.In announcing the approval, the FDA noted that prostate cancer is the second-leading cause of cancer death in U.S. men. Accurate staging is key for managing patients who have a suspected recurrence of cancer after primary treatment.
Current imaging tests aren't able to find the location of recurrent prostate cancer in patients with very low PSA levels, according to Dr. Libero Marzella, PhD, director of the Division of Medical Imaging Products in the FDA's Center for Drug Evaluation and Research.
"Axumin is shown to provide another accurate imaging approach for these patients," Marzella said in a press release.
In its regulatory submission for Axumin, Blue Earth provided results from two clinical studies evaluating the safety and efficacy of the radiopharmaceutical. In the first study of 105 men with suspected recurrent prostate cancer, the results of Axumin scans were compared with histopathology and biopsy. Radiologists interpreted the scans onsite initially, and then three independent, blinded radiologists read the scans.
In the second study, researchers evaluated the agreement between Axumin scans and studies acquired with another PET radiopharmaceutical, choline-11, in patients with median PSA values of 1.44 ng/mL. Again, onsite radiologists read the scans, and then the same three independent radiologists from the first study read the Axumin scans.
In both studies, the results of the independent scan readings were consistent with each other and confirmed the results of the onsite readings, the FDA noted. The studies also supported the safety and efficacy of Axumin.
The FDA accepted Blue Earth's regulatory filing of Axumin late last year under a priority review designation. The PETNet Solutions subsidiary of Siemens Healthineers has commercial rights to manufacture, distribute, and sell Axumin in the U.S.