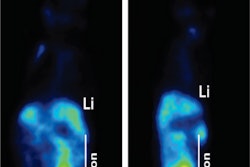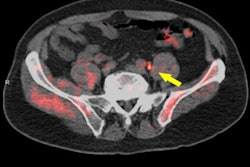
A study in the March issue of the Journal of Nuclear Medicine is touting the PET radiotracer fluciclovine F-18 for its ability to guide and monitor targeted treatment for recurrent prostate cancer.
Not only does the radiotracer allow for individualized, targeted therapy, but it does so with no demonstrated increase in early side effects from radiation therapy, said lead author Dr. Ashesh Jani from the Winship Cancer Institute of Emory University.
Fluciclovine was developed and is currently marketed by Blue Earth Diagnostics under the brand name Axumin; it received U.S. Food and Drug Administration approval in May 2016. Axumin is indicated for use in PET studies to identify suspected sites of prostate cancer recurrence in men who have elevated levels of prostate-specific antigen (PSA) following prior treatment.
In this three-year study, the researchers enrolled 96 patients who initially received treatment planning based on MR or CT abdominopelvic imaging for recurrent prostate cancer after prostatectomy. The Axumin scans with PET/CT resulted in modification of the treatment plans in 45 patients (47%) (JNM, March 2017, Vol. 58:3, pp. 412-418).
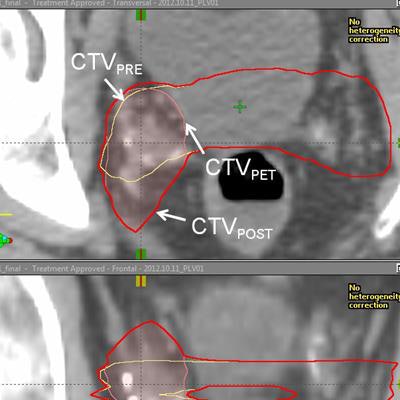
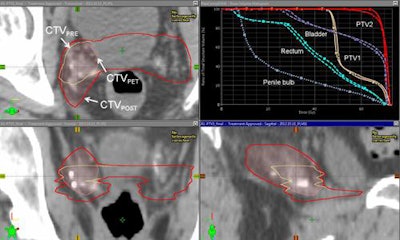 Representative example of target definition with fluciclovine-PET. Images courtesy of Dr. Ashesh Jani, Dr. David Schuster, and JNM.
Representative example of target definition with fluciclovine-PET. Images courtesy of Dr. Ashesh Jani, Dr. David Schuster, and JNM.There was no increase in bladder or rectal radiation dose or in acute genitourinary or gastrointestinal toxicity with Axumin, the researchers noted, although there was a higher radiation dose delivered to the penile bulb.
The findings could have meaningful implications beyond prostate cancer, Jani said. The methodology is applicable to other novel imaging agents and may lead to the improvement of cancer control outcomes.