PHILADELPHIA - With the addition of F-18 fluciclovine to PET/CT scans, clinicians changed the treatment regimen for a majority of men with recurrent prostate cancer and located previously undetected lesions in a new study, which was presented on Tuesday at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) conference.
The findings come from the LOCATE trial, fully known as the Impact of F-18 Fluciclovine PET/CT on Management of Patients With Rising Prostate-Specific Antigen After Initial Prostate Cancer Treatment. In the prospective, multicenter, open-label study conducted at 15 sites in the U.S., almost 60% of patients had their clinical management changed due to F-18 fluciclovine PET imaging, with approximately three-quarters of those treatment adjustments classified as a major change, the researchers reported.
"While investigation of the long-term clinical outcomes of these changes in management is warranted, these results indicate, for the first time in a prospective U.S. study, that decisions based on fluciclovine-PET/CT imaging may facilitate more appropriate management in men with biochemical recurrence of their prostate cancer," said lead author Dr. Austin Pantel from the University of Pennsylvania in a statement from the SNMMI. "The study also demonstrates the broad potential applicability of F-18 fluciclovine PET/CT imaging across a range of clinical settings."
The LOCATE trial measured the percentage of men with suspected biochemical recurrence of prostate cancer following initial prior therapy whose treatment plan changed after PET/CT scans with fluciclovine (Axumin, Blue Earth Diagnostics). Researchers also focused on the association between positive scan results and the clinical recurrence of the cancer, as well as prostate-specific antigen (PSA) levels and Gleason scores.
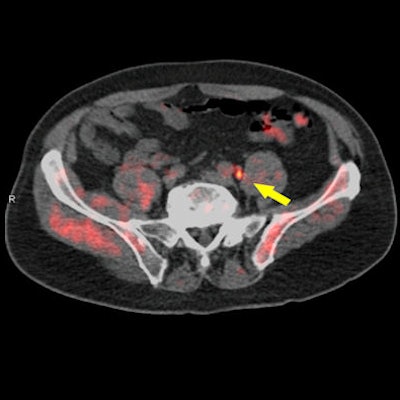
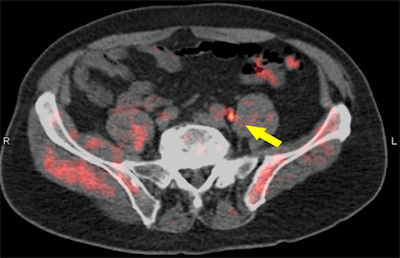 Fused fluciclovine-PET/CT image indicates recurrent prostate cancer (arrow) in a pelvic lymph node. Image courtesy of Dr. Barry Seigel, Mallinckrodt Institute of Radiology, and SNMMI.
Fused fluciclovine-PET/CT image indicates recurrent prostate cancer (arrow) in a pelvic lymph node. Image courtesy of Dr. Barry Seigel, Mallinckrodt Institute of Radiology, and SNMMI."Recurrent prostate cancer poses an important medical challenge," Pantel said. "Currently approved anatomical imaging procedures have limitations in identifying the sites of recurrence of prostate cancer after definitive treatment. This can make decision-making difficult when assessing these patients."
The study included 213 subjects who initially had negative or equivocal findings of recurrent prostate cancer on conventional imaging, such as a bone scan or CT or MR imaging. The median time from initial diagnosis was 4.5 years, while the median time since cancer recurrence was approximately six months. Questionnaires completed by treating physicians documented changes in management after the PET study.
The researchers found that 126 patients (59%) had their clinical management changed due to the fluciclovine-PET results. Among those changes, 98 men (78%) had major alterations in treatment strategy, including a change in modality. In addition, disease was detected in the prostate, pelvic tissue, abdominal lymph nodes, and, less commonly, bone. Interestingly, fluciclovine-PET influenced the treatment management of 36 (39%) of 91 men with negative scan results. In addition, there was no association between Gleason scores at diagnosis and positive scans.
The outcomes are particularly beneficial for the 30% or so of patients with prostate cancer who will develop local or distant recurrence within 10 years of radical prostatectomy or radiation therapy, according to Pantel and colleagues.
"Selecting appropriate treatment for men with recurrent prostate cancer is critical. Many options are available, and additional information, such as that provided by F-18 fluciclovine PET/CT, may help tailor personalized treatment plans," he said. "Clinical studies, such as the LOCATE trial, are important to investigate the role of the current generation of molecular imaging agents in guiding management for men with recurrent prostate cancer. This study highlights the key role of nuclear medicine in directing effective patient management and care."
Study disclosure
The LOCATE trial was sponsored by Blue Earth Diagnostics.