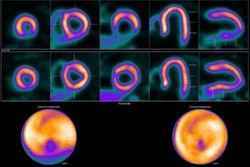
Cardiovascular Associates of America (CVAUSA) plans to expand its adoption of GE HealthCare’s (GEHC) U.S. Food and Drug Administration (FDA)-approved cardiac PET radiotracer Flyrcado (flurpiridaz F-18).
CVAUSA currently performs approximately 85,000 cardiac PET procedures each year. The Arlington Heights, IL-based network plans to extend the rollout across 25 sites, it said.
Results from a clinical pilot that evaluated Flyrcado in the real-world setting of a high-volume practice further validated the tracer’s role in cardiac PET imaging, GEHC added. In addition, flurpiridaz F-18 is featured in a new international procedure standard developed jointly by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), the American Society of Nuclear Cardiology (ASNC), the European Association of Nuclear Medicine (EANM), and the American College of Nuclear Medicine (ACNM), which was published in October in the Journal of Nuclear Medicine.