French molecular imaging firm Advanced Accelerator Applications (AAA) said the U.S. Food and Drug Administration (FDA) has approved a new drug application for its Lutathera radiopharmaceutical.
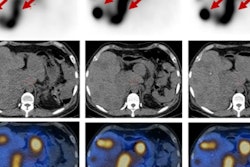
Lutathera (lutetium-177 DOTATATE) was approved for the radioactive treatment in adults of somatostatin-positive gastroenteropancreatic neuroendocrine tumors, including foregut, midgut, and hindgut tumors, AAA said.
Approval came following a phase III trial known as NETTER-1. In the study, researchers compared peptide receptor radionuclide therapy (PRRT) using Lutathera with the standard of care for patients with inoperable midgut neuroendocrine tumors. Lutathera therapy led to a 79% greater reduction in risk of disease progression or death than standard treatment, according to the group.
Lutathera previously received orphan drug status from the FDA and the European Medicines Agency (EMA), AAA said.