PHILADELPHIA - Researchers from the U.S. and China are reporting early success with a new, long-lasting theranostic radionuclide therapy for patients with advanced metastatic neuroendocrine tumors (NETs). Study results were presented on Sunday at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting.
The phase I trial featured the pairing of the therapeutic agent lutetium-177 (Lu-177) DOTATATE (Lutathera, Advanced Accelerator Applications) with the diagnostic agent gallium-68 (Ga-68) DOTATATE (Netspot, Advanced Accelerator Applications) to create Lu-177 DOTA-EB-TATE. The theranostic agent is designed to locate and mark NET lesions for targeted peptide receptor radionuclide therapy (PRRT). Because PRRT binds to cancer-related somatostatin receptors (SSTRs) on the tumor cells, healthy cells remain unharmed.
"Lu-177 DOTA-EB-TATE showed remarkably higher uptake and retention in NET tumors as well as significantly increased accumulation in the kidneys and red marrow," wrote lead author Dr. Jingjing Zhang, from Peking Union Medical College Hospital in China, and colleagues in an abstract in the Journal of Nuclear Medicine (April 13, 2018). "It has great potential to be used in PRRT for NET tumors with lower dose and less frequency of administration."
Lutathera was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of NETs. The radiolabeled somatostatin analogues, or peptides, in Lutathera quickly clear from the blood through the kidneys, however, limiting the accumulation of radioactivity within tumors and necessitating additional treatment cycles to provide the therapeutic dose.
For this study, the researchers from Peking and the National Institutes of Health (NIH) enrolled six men and two women (age range: 27-61 years) with advanced metastatic neuroendocrine tumors. Each patient underwent whole-body Ga-68 DOTATATE PET/CT. Five patients were later given a single intravenous injection (0.35-0.70 GBq) of Lu-177 DOTA-EB-TATE within one week. The researchers then imaged the subjects at two, 24, 72, 120, and 168 hours using SPECT/CT. The other three patients received a single dose (0.28-0.41 GBq) of Lu-177 DOTATATE and underwent SPECT/CT scans at one, three, four, 24, and 72 hours.
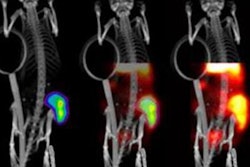
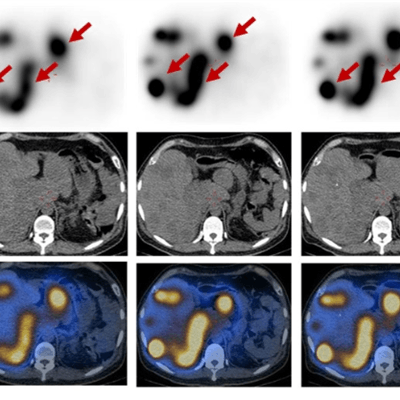
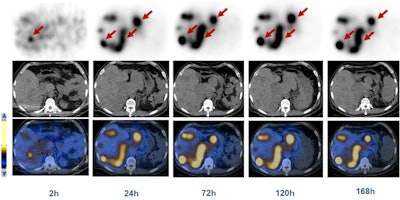 SPECT/CT shows a 45-year-old man with advanced NETs and multiple liver metastases. Scans were performed at two, 24, 72, 120, and 168 hours after the administration of Lu-177 DOTA-EB-TATE. The radiopharmaceutical cleared from the blood pool over time and was retained in the tumors (arrows). Images courtesy of Zhang et al and SNMMI.
SPECT/CT shows a 45-year-old man with advanced NETs and multiple liver metastases. Scans were performed at two, 24, 72, 120, and 168 hours after the administration of Lu-177 DOTA-EB-TATE. The radiopharmaceutical cleared from the blood pool over time and was retained in the tumors (arrows). Images courtesy of Zhang et al and SNMMI.In addition, the researchers performed complete physical exams on the subjects, recording vital signs, blood counts, and biochemistry and immunology analyses immediately before treatment; at one, three, and seven days after treatment; and again at three months after treatment. They discovered that Lu-177 DOTA-EB-TATE was well-tolerated by the NET patients, with no reported adverse effects during the procedure and follow-up.
Furthermore, Lu-177 DOTA-EB-TATE showed extended circulation in the blood and achieved a 7.9-fold increase in tumor dose delivery, compared with Lu-177 DOTATATE. There were also significant dose delivery increases to the kidneys (3.2-fold increase) and bone marrow (18.2-fold increase) in patients receiving Lu-177 DOTA-EB-TATE, compared with those receiving Lu-177 DOTATATE.
"This long-lasting radiolabeled somatostatin analogue has remarkably enhanced uptake and retention in SSTR-positive tumors, which is important to increase the therapeutic efficacy in patients," Zhang said in a press release. "With proper selection of patients with advanced metastatic neuroendocrine tumors, Lu-177 DOTA-EB-TATE has great potential to be a highly effective treatment, while providing a safe dose with less frequency of administration than is possible with Lu-177 DOTATATE."