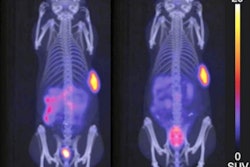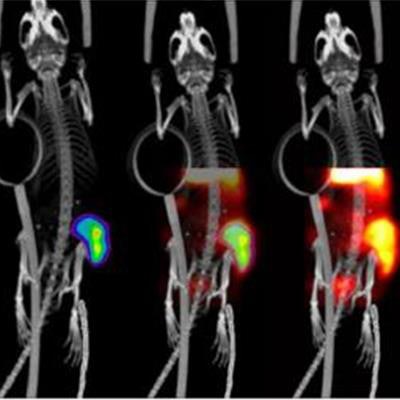
Researchers from the U.K. may have developed a way to observe in vivo DNA damage a few days after treatment for neuroendocrine tumors with lutetium-177 (Lu-177)-DOTATATE, according to a proof-of-concept preclinical study published in the May issue of the Journal of Nuclear Medicine.
The key component is the SPECT imaging agent indium-111 (In-111)-antiγH2AX-TAT, which would allow clinicians to visualize the DNA damage response marker known as γH2AX. By doing so, physicians potentially could assess the efficacy of Lu-177-DOTATATE radionuclide therapy within three days of treatment and, if necessary, adjust the regimen accordingly.
"Understandably, some challenges need to be overcome before translation to the clinic is possible," wrote the authors, led by Edward O'Neill, PhD, postdoctoral research fellow in the department of oncology at the University of Oxford. "Our initial results here were obtained using athymic mice bearing rat xenografts, but the results can be readily extrapolated to the human situation, given that similar interplay exists between Lu-177 uptake, heterogeneous Lu-177 tumor uptake, and DNA damage and repair."
Among the molecular radiotherapy options for patients with neuroendocrine cancer is the radiation-emitting combination of the therapeutic medical isotope Lu-177 and the radionuclide DOTATATE. DOTATATE also can be radiolabeled with In-111 for SPECT imaging. While Lu-177-DOTATATE treatment has shown that it can extend progression-free survival, the degree to which the regimen could cause adverse radiobiologic effects remains unknown.
"In our study, we sought to image the molecular biological effects of Lu-177-DOTATATE radionuclide therapy by visualizing the DNA double-strand break damage response marker γH2AX," explained study co-author Bart Cornelissen, PhD, associate professor in the university's department of oncology, in a statement. "One strategy to develop this metric is to determine if sufficient damage has been afflicted to the tumor, which would allow treating physicians to tailor subsequent doses to ensure therapeutic success."
To achieve that goal, the researchers exposed six cancer cell lines to external-beam radiation therapy (EBRT) or Lu-177-DOTATATE and then measured the number of γH2AX foci and their survival, which would indicate the extent of DNA damage. Mice with the same cell line were treated with Lu-177-DOTATATE or given an ineffective fake treatment.
SPECT/CT images were acquired at one, 24, 48, and 72 hours after administration for approximately 10 minutes using a single-gantry SPECT/CT and PET/CT preclinical scanner (Vector4CT, MILabs). Immediately after the first SPECT imaging session, the mice were given In-111-anti-γH2AX-TAT or In-111-IgG-TAT (JNM, May 2020, Vol. 61:5, pp. 743-750).
O'Neill and colleagues found uptake of Lu-177-DOTATATE in all six cancer cell lines in vitro, as well as increased γH2AX foci and decreased survival of the foci further validating the molecular radiotherapy strategy. Most importantly, the γH2AX foci, which were highlighted by Lu-177-DOTATATE in the mice, "were successfully imaged by SPECT in vivo using In-111-anti-γH2AX-TAT," the researchers noted, with sustained γH2AX signal accumulation over the three-day course of Lu-177-DOTATATE administration, which would indicate DNA damage to the mice.
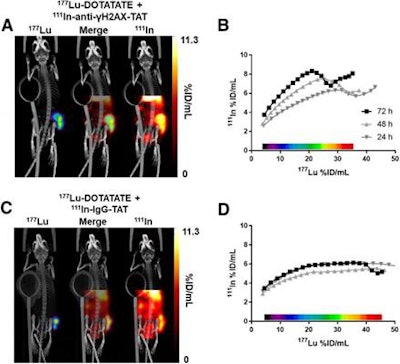 Dual-isotope SPECT/CT images (A) of mice 71 hours after intravenous administration of In-111-anti-γH2AX-TAT (5 MBq) and 72 hours after intravenous administration of Lu-177-DOTATATE (20 MBq). Tumor is indicated by purple contour in Lu-177 image. Correlation between In-111 and Lu-177 signal (B) in tumor volume in voxel collections based on Lu-177 signal quantification in SPECT image of animal in A. Dual-isotope SPECT/CT images (C) of mice after administration of In-111-IgG-TAT (5 MBq) and Lu-177-DOTATATE (20 MBq). Tumor is indicated by purple contour in Lu-177 image. Correlation between In-111 and Lu-177 signal (D) in tumor volume in voxel collections based on Lu-177 signal quantification in SPECT image of animal in C. Images courtesy of O'Neill et al and Journal of Nuclear Medicine.
Dual-isotope SPECT/CT images (A) of mice 71 hours after intravenous administration of In-111-anti-γH2AX-TAT (5 MBq) and 72 hours after intravenous administration of Lu-177-DOTATATE (20 MBq). Tumor is indicated by purple contour in Lu-177 image. Correlation between In-111 and Lu-177 signal (B) in tumor volume in voxel collections based on Lu-177 signal quantification in SPECT image of animal in A. Dual-isotope SPECT/CT images (C) of mice after administration of In-111-IgG-TAT (5 MBq) and Lu-177-DOTATATE (20 MBq). Tumor is indicated by purple contour in Lu-177 image. Correlation between In-111 and Lu-177 signal (D) in tumor volume in voxel collections based on Lu-177 signal quantification in SPECT image of animal in C. Images courtesy of O'Neill et al and Journal of Nuclear Medicine."The application of this imaging technique could provide a very early indicator of tumor damage without having to wait for changes in tumor volume, which currently may take months to find out," O'Neill added in a statement. "When using therapeutic response assessment with molecular imaging, making rapid decisions becomes possible, including dose reduction to avoid side effects, assessment of combination therapies, or -- in the absence of any measurable response -- initiation of palliative options designed toward improving quality of life."