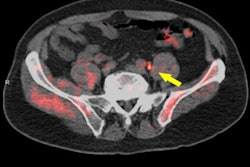
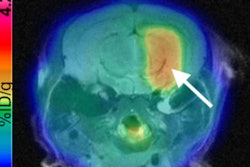
Radiopharmaceutical developer FluoroPharma Medical has acquired an exclusive option to license the meta-fluorobenzylguanidine (MFBG) imaging agent from Ground Fluor Pharmaceuticals.
MFBG, labeled with the isotope F-18, can be used in conjunction with PET for assessing neuronal integrity. It may play an important role in the diagnosis and assessment of various cancers, as well as some aspects of cardiovascular disease, according to the firm.
Ground Fluor Pharmaceuticals established a company-sponsored investigational new drug application and plans to move MFBG into late-stage development in the coming months, with the expectation of U.S. Food and Drug Administration (FDA) approval in 2020. FluoroPharma will work with Ground Fluor to move the agent into routine clinical use.