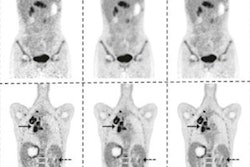
Imaging artificial intelligence (AI) start-up Subtle Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its SubtlePET AI algorithm.
SubtlePET is designed to help hospitals and imaging centers enhance PET images acquired from shorter scan times, yielding an improved patient experience, faster exam throughput, and higher profitability for providers, according to the vendor. The algorithm is being piloted at a number of facilities in the U.S. and around the world. Subtle Medical also recently obtained the CE Mark to market SubtlePET in Europe.
The company is also developing other AI products such as its SubtleMR algorithm for accelerating MRI scans and its SubtleGAD software for reducing gadolinium dose during MR imaging. Both algorithms are currently undergoing clinical evaluation, Subtle Medical said.