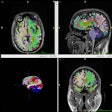
MRI computer-aided detection developer 3TP of Southampton, NY, has received Food and Drug Administration clearance for its contrast-enhanced MRI CAD technology.
The product measures tissue permeability and extracellular volume fraction on a pixel-by-pixel basis at three distinct time points. It’s based on a model developed by Hadassa Degani of the Weizmann Institute of Science, in Rehovot, Israel. The software assigns a color to each pixel, enabling a radiologist to rapidly identify conspicuous lesions, 3TP said.
The initial application for the software is for noninvasive breast imaging. The process is currently being researched for application to prostate and lung tissue analysis, according to the firm. The company plans to initiate distribution of the product this year.
By AuntMinnie.com staff writers
March 11, 2004
Related Reading
MRI perfusion combo technique addresses urologic staging in prostate cancer, May 15, 2003
Copyright © 2004 AuntMinnie.com


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)