(Booth 6754) Breast MRI developer Aurora Imaging Technology of North Andover, MA, will highlight a new biopsy capability for its 1.5-tesla dedicated breast scanner.
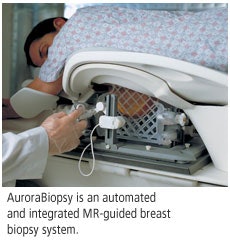
AuroraBiopsy can also target multiple lesions in the same breast or both breasts in a single procedure. It also offers physicians a large access area to the breasts for both medial and lateral approaches, and includes integrated breast stabilization paddles that allow access to sample all breast sizes.
Aurora has also worked with needle device manufacturers to develop a customized needle-guidance procedure for AuroraBiopsy that accommodates all current core or vacuum-assisted biopsy needle devices currently on the market, according to the company.
AuroraBiopsy has received 510(k) clearance from the U.S. Food and Drug Administration, and is available in the U.S. and international markets.