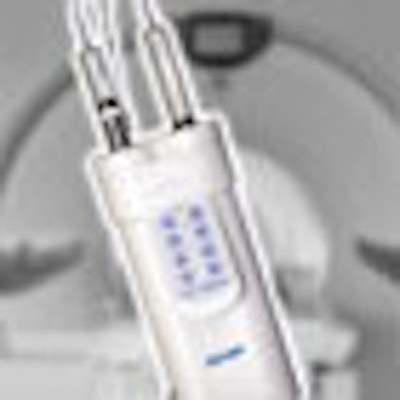
(Booth 4720) At last year's RSNA show, E-Z-EM launched its first product for the MRI market, EmpowerMR, as a work-in-progress. Since then, the Lake Success, NY, company has received U.S. Food and Drug Administration 510(k) clearance for the injector, and has begun commercial sales.
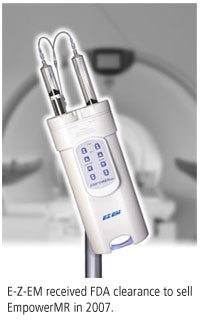
In addition, EmpowerMR's dual head supports contrast and saline flush, and its tilt sensor/lockout helps minimize the risk of air embolism. It also has a color-coded illuminating handle that's readable from a distance, with different colors displayed depending on whether saline or contrast is being injected. A dual-control feature allows EmpowerMR to be operated remotely or directly from the MRI scanner.
On the information technology side, E-Z-EM will show IRiS 2.0, the newest version of the company's injector reporting system software. IRiS works with the company's Empower CT and MR contrast injectors, and enables users to track factors such as patient ID, creatinine values, glomerular filtration rate (GFR) measurements, type of contrast used, and number of contrast extravasations.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)





.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











