(Booth 7570) Italian CAD developer im3D of Turin will appear at radiology's fall showcase in Chicago with a new breast MRI application, in addition to its flagship CAD software for CT colonography.
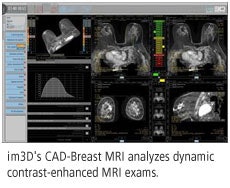
im3D believes that CAD-Breast MRI can also help clinicians monitor the effectiveness of therapy by working at the vascular level to process and analyze DCE-MRI results. Other features include automatic detection and segmentation of suspicious areas, extraction of parametric maps of physiological characteristics, and the ability to interface with almost any MRI device, according to the company.
The software will be shown as a work-in-progress and has not received U.S. Food and Drug Administration clearance. Commercialization is expected in late 2008.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)





.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)