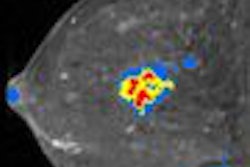
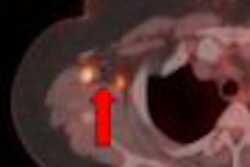
Norwegian functional MRI developer NordicNeuroLab (NNL) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its nordicAktiva software.
The Bergen, Norway-based firm's product features step-by-step guidance through preparation and stimulus presentation and integrates with the NNL Hardware system and automated data analysis through its nordicIce Bold module.
Related Reading
NordicNeuroLab forms U.S. office, April 16, 2008
NordicNeuroLab wins clearance for fMRI system, January 4, 2008
Copyright © 2008 AuntMinnie.com