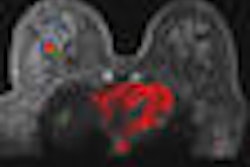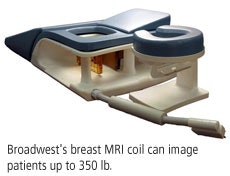
The coil can image a patient up to 350 lb and features patient positioning aids that can customize the coil's configuration. It is designed for a variety of clinical indications in breast disease management, including searching for primary breast cancer in women with a positive axillary node; staging of tumor extent in women with known breast cancer; searching for multifocal, multicentric, or bilateral breast cancer in women with known breast cancer; evaluating for tumor recurrence after surgery or radiation; and screening high-risk women.
The coil and software have been approved by the U.S. Food and Drug Administration for compatibility with Siemens scanners; the package's interventional components and software are not yet approved.