Dear AuntMinnie Member,
The U.S. Food and Drug Administration (FDA) issued a warning on Wednesday of a possible link between breast implants and a rare form of cancer.
A review by the agency indicated that there could be a link between breast implants and anaplastic large cell lymphoma (ALCL), a rare cancer of the immune system that strikes an estimated one in 500,000 women each year in the U.S. The FDA said it found 34 published cases of ALCL in women with implants.
The agency couched its warning by saying that it believes that any potential risk is low; still, it cautioned women with implants and their physicians to be aware of the disease, particularly patients with late-onset persistent peri-implant seroma, capsular contracture, or masses adjacent to the breast implant.
Learn more by clicking here, or visit our Women's Imaging Digital Community at women.auntminnie.com.
VA investigates MRI safety
In other regulatory news, the U.S. Department of Veterans Affairs (VA) this week released a new report on MRI safety at a number of its facilities, according to an article we're highlighting in our MRI Digital Community.
The VA commissioned an investigation after an incident in which a patient at one of its facilities had to crawl out of an MRI scanner when the system's panic button didn't work.
After reviewing MRI safety practices at a sample of VA sites, the agency concluded that while MRI sites are generally following guidelines, some improvements could be made. Find out what they are by clicking here.
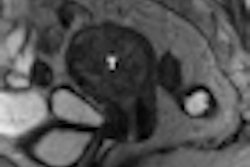
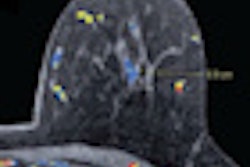
In other news in the community, learn about researchers in New York City who used MRI to determine the anatomic origin of uterine cancer -- a key finding for accurate treatment planning. Get the rest of the story by clicking here.
Also, learn about one institution's experience when it switched from one gadolinium-based MRI contrast agent to another in an article available by clicking here.
You can also find these stories by visiting the community at mri.auntminnie.com.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











