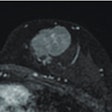
Women with silicone breast implants may not need regular follow-up with MRI, despite the U.S. Food and Drug Administration (FDA) recommendation that women with these implants undergo the scans in the years after surgery to screen for implant rupture, according to a study published in the March issue of Plastic and Reconstructive Surgery.
Dr. Jae Song and colleagues at the University of Michigan reviewed 21 studies evaluating MRI and/or ultrasound to detect rupture of silicone breast implants. They found that MRI was 14 times more likely to detect problems in women with implant-related symptoms than in women without symptoms. Because most of the women in the studies had symptoms, the true accuracy of MRI for detecting implant-related problems in asymptomatic women was likely much lower, according to the researchers.
The study raises important questions about the accuracy of MRI for follow-up in women with implants, especially in women without symptoms, Song and colleagues wrote. Song's team noted that in reported cases of implant rupture, the average age of the implants is more than 10 years, leading the researchers to conclude that the benefits of screening for implant rupture within the first 10 years after surgery are unclear.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)