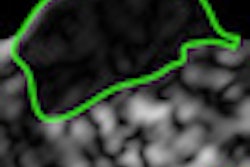
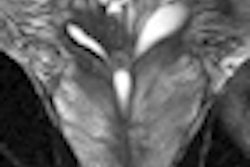
A technique in which MRI scans are fused with transrectal ultrasound images to guide biopsies may replace systematic biopsies to improve diagnoses for patients with prostate cancer, as well as aid those with a small amount of low-grade cancer undergoing surveillance.
Researchers from the University of California, Los Angeles (UCLA) have found that the combination of MRI and transrectal ultrasound can locate additional cancer and help patients avoid some of the discomfort and risks associated with systematic biopsies.
High false-negative rate
Currently, prostate cancer is detected by physical exams and measuring serum levels of prostate-specific antigen (PSA) as a marker of prostate cancer, followed by ultrasound-guided sextant needle biopsy. Previous research, however, has found that these methods can produce false-negative results in as many as 30% of cases.
"MRI has been shown to identify cancer in more than half of men where the initial biopsies are negative, but direct MRI-guided biopsy is not universally available," said lead author Dr. Daniel Margolis, assistant professor of radiology at UCLA's David Geffen School of Medicine, who presented study results at the recent International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting. "The ability of ultrasound-guided fusion to biopsy MRI-delineated targets has been elucidated, and we wanted to show the yield of MRI/ultrasound-guided fusion biopsies."
Margolis and colleagues enrolled 54 consecutive men, screened the subjects for serum levels of PSA, and provided physical exams. If the results were abnormal, patients underwent a prostate MRI exam, followed by both systematic and targeted transrectal ultrasound-guided biopsies. Based on those findings, patients were referred for surgery, radiation therapy, or active surveillance if small amounts of low-grade disease were identified.
MR imaging was performed with a 3-tesla system (Magnetom Trio, Siemens Healthcare) using an external phased-array coil. The researchers performed diffusion-weighted imaging (DWI) with an apparent diffusion coefficient (ADC) map and dynamic contrast-enhanced (DCE) perfusion in addition to T2-weighted imaging.
The 54 men also underwent ultrasound-guided biopsies using MRI/ultrasound fusion software (Artemis, Eigen) in addition to standard biopsies.
Cancer targets
Targets were chosen based on either decreased T2 signal or abnormally decreased ADC or abnormal DCE. Margolis and colleagues scored the fused images from the T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast-enhanced imaging on a scale of one to five.
The results yielded 86 potential targets in 49 patients, with 61 (71%) of the 86 targets biopsied in 40 patients. "The other targets were considered low probability and were deferred," Margolis said.
The researchers also added parallax imaging to the ultrasound targeting after the first 25 patients. In nine patients, the biopsy was converted to freehand ultrasound, in large part due to patient motion, which caused misregistration of the images.
"In terms of our [diagnostic] yield, biopsies were positive in 14 targets," Margolis said. "On a per-target basis, 23% were positive, but 47% were positive after the use of parallax imaging."
In 14 patients, biopsies were positive only on the systematic course. However, the majority of these biopsies had less than 1 mm of cancer, so there was a small volume of low-grade disease. Five lesions were in the same location as the target, Margolis and colleagues noted. These findings were all prior to the use of parallax imaging.
"Our conclusion is that MRI/ultrasound fusion for targeted biopsies finds additional cancers compared to systematic biopsies and may replace systematic biopsies, resulting in fewer total biopsies, improved yield, and improved confidence for active surveillance," Margolis said.