With the help of an MRI biomarker, researchers have found that people with smaller regions of the brain's cortex may be more likely to develop symptoms consistent with early Alzheimer's disease, even if they currently have no memory problems.
The study's findings, published online December 21 in the journal Neurology, could help detect people at high risk of preclinical Alzheimer's disease and future cognitive decline, according to a group led by Dr. Bradford Dickerson at Massachusetts General Hospital.
The researchers used data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) to select 159 cognitively normal individuals with an average age of 76. At the beginning of the study and over the next three years, the participants were given tests that measured memory, problem solving, and the ability to plan and pay attention.
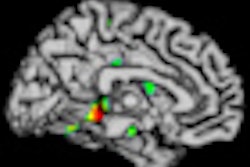
All subjects also underwent a 1.5-tesla MRI exam to serve as a baseline measure. The imaging biomarker used was the Alzheimer's disease signature (ADsig) of regional cortical thinning, which previous studies have shown to be a reliable marker of mild Alzheimer's disease and useful for predicting Alzheimer's in individuals with mild cognitive impairment. The Alzheimer's disease signature is also detectable in cognitively normal individuals with brain beta-amyloid as measured by PET imaging.
Dickerson and colleagues also recently found that ADsig is detectable in cognitively normal adults who later develop Alzheimer's. Individuals who have this biomarker have a three times greater risk of developing Alzheimer's over the next decade.
To determine the Alzheimer's disease signal, the researchers measured the average thickness of nine regions of interest in the cerebral cortex. Each individual was then classified as follows: ADsig-low if the value was one standard deviation or more below the mean, ADsig-average if it was within one standard deviation of the mean, and ADsig-high if it was one standard deviation or more above the mean.
Individuals in the ADsig-low group "were considered to have evidence of early neurodegeneration, which is consistent with Alzheimer's, and therefore were considered as high risk for preclinical Alzheimer's," Dickerson and colleagues noted.
Of the 159 cognitively normal subjects, 19 were found to be at high risk (ADsig-low) for preclinical Alzheimer's disease using the MRI biomarker. Of the remaining individuals, 116 were classified as ADsig-average and 24 were considered ADsig-high.
During the three years of follow-up after MRI, cognitive decline developed in 21% of the high-risk group, 7% of the average-risk group, and none of the low-risk group.
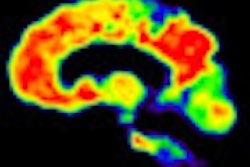
In the measurement of brain size, subjects who experienced cognitive decline had a mean cortical thickness of 2.41 mm, which was significantly smaller than the cortical thickness of 2.56 mm among those who were cognitively stable.
The researchers also found that 60% of the group considered most at risk for early Alzheimer's disease had abnormal levels of proteins associated with the disease in cerebrospinal fluid, compared with 36% of individuals at average risk and 19% of participants who were at low risk.
Study limitations
Dickerson and colleagues cited the relatively small number of individuals and the short follow-up period as study limitations. "The apparently low sensitivity of the present MRI biomarker to cognitive decline is likely related in part to the short follow-up interval," the authors wrote.
"Further research is needed on how using MRI scans to measure the size of different brain regions in combination with other tests may help identify people at the greatest risk of developing early Alzheimer's as early as possible," Dickerson added in a statement on the study.
In an accompanying editorial published in Neurology, Susan Resnick, PhD, of the National Institute on Aging, wrote that the "ability to identify people who are not showing memory problems and other symptoms, but may be at a higher risk for cognitive decline, is a very important step toward developing new ways for doctors to detect Alzheimer's disease."



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)

.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











