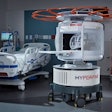
Toshiba America Medical Systems has received clearance from the U.S. Food and Drug Administration for its new Vantage Titan 1.5-tesla MR series of scanners, which includes eight-, 16-, and 32-channel MR systems.
Titan eight-channel features a 71-cm bore, Toshiba's Pianissimo noise-reduction technology, noncontrast imaging protocols, integrated coils with Octave Speeder technology, and an M-Power user interface, according to the firm.
For enhanced cardiac imaging, Titan can be upgraded to a 16- or 32-channel system, which allows higher spatial and temporal resolution, Toshiba said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)