Medical device developer Monteris Medical said the first-in-human study on the company's NeuroBlate thermal therapy system has produced promising results
Early indications are that the NeuroBlate system appears to provide a new, safe, and minimally invasive way to treat recurrent glioblastoma, according to the vendor.
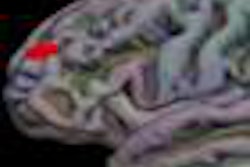
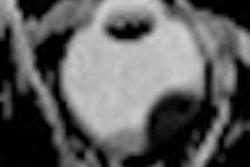
The phase I clinical trial, the results of which appeared online April 5 in the Journal of Neurosurgery, treated 10 patients with the specially designed laser probe system. The patients, who had a median age of 55, had tumors that were diagnosed to be inoperable or high-risk for open surgical resection, because their locations were close to vital areas in the brain or difficult to access with conventional surgery, Monteris said.
In a statement, the study authors reported the NeuroBlate procedure was well-tolerated, and all 10 patients were alert and responsive within one to two hours after the procedure, with nine of 10 patients ambulatory within hours. Response and survival aslo was nearly 10.5 months, better than expected for patients with such advanced disease, the authors said.
Lead study author Dr. Andrew Sloan and principal investigator Dr. Gene Barnett, directors of the Brain Tumor and Neuro-Oncology Center at University Hospitals Case Medical Center and Case Comprehensive Cancer Center, are paid consultants for Monteris and members of the company's medical advisory board.
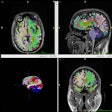
NeuroBlate uses a minimally invasive, MRI-guided laser system to coagulate or heat and kill brain tumors. The procedure is conducted in an MRI system, enabling surgeons to plan, steer, and see in real-time the device, the heat map of the area treated by the laser, and the tumor tissue that has been coagulated, Monteris said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)