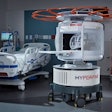
Biomedical device company Biotronik has received approval from the U.S. Food and Drug Administration (FDA) to expand its ProMRI trial.
The third phase of the trial will study the company's ProMRI technology in implantable cardioverter-defibrillator (ICD) devices for use in MRI scanners, Biotronik said.
The first phase of the ProMRI trial evaluated the safety of the Biotronik Entovis pacemaker systems during MRI scans, excluding scans in the chest area. The phase was completed on November 2013, according to the company.
In that same month, the FDA approved the second phase of the study, which expanded the trial to evaluate the safety of the company's pacemaker systems during MRI scans, including cardiac and thoracic spine scans.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)