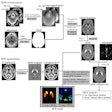
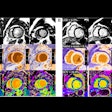
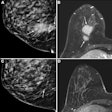
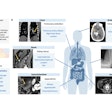
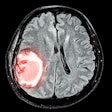
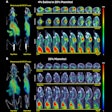
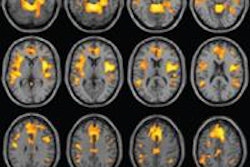
The U.S Food and Drug Administration (FDA) is proposing to revise the information vendors should include in their 510(k) premarket submissions for MRI diagnostic devices (MRDDs).
The draft guidance, titled "Submission of Premarket Notifications for Magnetic Resonance Diagnostic Devices," is designed to expand upon the current general requirements and would be used in conjunction with general information regarding the content and format of a 510(k) premarket notification.
MRDDs are class II medical devices that require premarket notification and an agency determination of substantial equivalence prior to marketing, according to the FDA.
The agency is now taking feedback on the proposal.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)