A team at the University of Maryland has developed a new strategy for facilitating drug penetration into the brain, according to a study published September 2 in Radiology.
In a study in mice, MR and PET imaging showed that adding sodium chloride (NaCL) to the standard osmotic agent mannitol safely increased blood-brain barrier permeability, noted lead author Guanda Qiao, MD, and colleagues.
“Combining 4% NaCl with 25% mannitol showed promise for improving the safe delivery of therapeutic agents to the brain through the endovascular route,” the group wrote.
The blood-brain barrier (BBB) protects the brain from harmful substances while allowing essential molecules to pass through. Infusing patients with 25% mannitol opens tight junctions in the BBB (via osmosis) and improves drug delivery, the researchers explained. The method has been in use since the 1980s, yet its effectiveness varies among patients and it can cause adverse effects if infusion rates are too high, the group added.
Thus, in this study, the researchers aimed to determine whether adding another known osmotic agent, NaCL (salt), to 25% mannitol could increase osmotic power. They assessed whether the approach could increase BBB opening for small molecules using gadolinium-based contrast agent–enhanced MRI at 9.4 tesla and for large molecules, PET/CT with zirconium-89 (Zr-89) bevacizumab-deferoxamine (antibody).
According to the results, in 44 mice, contrast-enhanced MRI showed that a combination of 25% mannitol and 4% NaCl opened the BBB more effectively than 25% mannitol alone; specifically, the BBB opening area ratio increased by 43%.
PET imaging showed significantly greater uptake of Zr-89 bevacizumab-deferoxamine in the ipsilateral hemisphere of the mice injected with mannitol and NaCL versus mannitol alone, with a mean standardized uptake value of 1.59 versus 0.51 (p=0.002) and maximum standardized uptake value of 2.77 versus 1.01 (p=0.008). PET imaging also provided spatial data on antibody biodistribution, showing that 4% saline in 25% mannitol enhanced delivery to the anterior brain regions compared with mannitol alone.
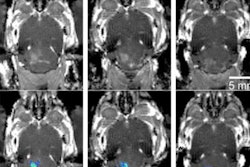
 PET/CT images in mice that underwent osmotic blood-brain barrier opening with (A) 4% NaCl in 25% mannitol or (B) 25% mannitol, followed by intra-arterial (IA) injection of zirconium-89 bevacizumab-deferoxamine and whole-body PET/CT with three-dimensional (3D) reconstruction. Coronal sections from left to right are ordered from anterior (A) to posterior (P); horizontal sections are ordered from superior (S) to inferior (I); and sagittal sections are ordered from left (L) to right (R). RSNA
PET/CT images in mice that underwent osmotic blood-brain barrier opening with (A) 4% NaCl in 25% mannitol or (B) 25% mannitol, followed by intra-arterial (IA) injection of zirconium-89 bevacizumab-deferoxamine and whole-body PET/CT with three-dimensional (3D) reconstruction. Coronal sections from left to right are ordered from anterior (A) to posterior (P); horizontal sections are ordered from superior (S) to inferior (I); and sagittal sections are ordered from left (L) to right (R). RSNA
Lastly, T2-weighted MRI scans obtained on days three and seven after injection with the osmotic agents revealed no abnormal findings, such as edema, stroke, inflammation, or microhemorrhages, in either group, the researchers noted.
“MRI and PET showed that the combination of two osmotically active agents, 25% mannitol and 4% NaCl, safely increased blood-brain barrier permeability to therapeutic antibodies,” the group wrote.
Ultimately, the results are encouraging and may inspire other research groups to explore enhanced BBB opening through osmotic pressure modulation, the researchers concluded.
In an accompanying editorial, Olivier Clement, MD, PhD, and Anne-Laure Gaultier, MD, both of Assistance Publique–Hôpitaux de Paris, wrote that the results need additional confirmation in other species (larger animals and humans) and in disease models where the BBB is not intact. The passage of other types of molecules than a small gadolinium chelate or a large antibody will also be needed, they added.
This further research could help start multicenter clinical trials, Clement and Gaultier suggested.
“Therapeutic applications would be in oncology first (high-grade gliomas and central nervous system lymphomas), but neurodegenerative diseases could also benefit from it with enzyme therapies targeting tau or gene therapy vectors to reach affected basal ganglia regions,” they concluded.
The full article is available here.