Low-intensity pulses of MRI-guided focused ultrasound (MRgFUS) could become a viable strategy to open areas of the blood-brain barrier and allow for targeted drug and stem-cell treatment for Alzheimer's disease, according to a study presented Tuesday at RSNA 2019 in Chicago.
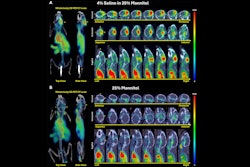
The success of the MRgFUS procedure was seen in follow-up MRI brain scans, which confirmed the blood-brain barrier opened within the target areas immediately after treatment and closed within 24 hours.
The blood-brain barrier consists of a network of blood vessels and tissues that keep foreign substances from entering the brain. The blocking creates a challenge to both researchers and clinicians looking to administer therapeutic treatment to key regions of the brain associated with cognitive impairment.
Preclinical studies have shown that MRgFUS can reversibly open the blood-brain barrier and allow for therapy delivery in animals. The question is: Can this procedure have the same beneficial effect on humans?
To that end, Dr. Ali Rezai, the director of the West Virginia University (WVU) Rockefeller Neuroscience Institute in Morgantown, WV, led researchers from three institutions in the clinical trial presented at RSNA 2019. Rezai and colleagues delivered MRgFUS to specific sites associated with memory function in the brains of three women, ages 61, 72, and 73. All three subjects were diagnosed with early-stage Alzheimer's disease and had evidence of amyloid plaques, which are linked to cognitive decline as they accumulate and spread.
To perform MRgFUS, a helmet with more than 1,000 separate ultrasound transducers angled in different orientations is placed over the patient's head after they are positioned in the MRI scanner. Each transducer delivers sound waves targeted to a specific area of the brain. The three women received three successive treatments at two-week intervals. Researchers monitored them for bleeding, infection, and fluid buildup.
MRI brain scans performed immediately after MRgFUS revealed that the blood-brain barrier opened within the target areas of the brain and closed within 24 hours.
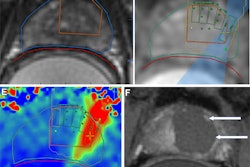
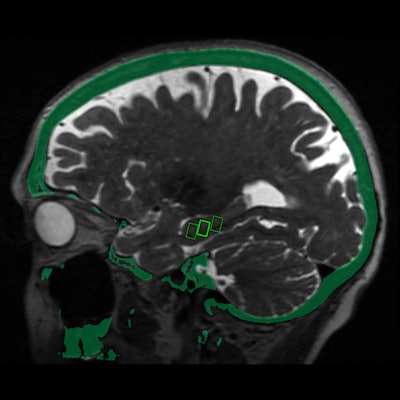
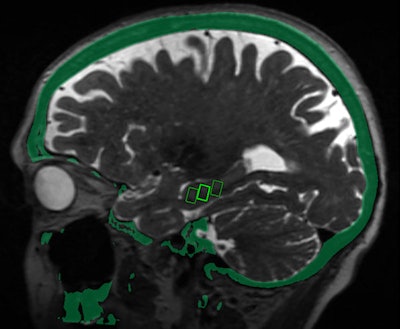 Fused MRI and CT image shows the three targeted sites in the brain's hippocampus, which provides a person's memory function and is adversely affected by Alzheimer's disease. Image courtesy of the RSNA.
Fused MRI and CT image shows the three targeted sites in the brain's hippocampus, which provides a person's memory function and is adversely affected by Alzheimer's disease. Image courtesy of the RSNA."We were able to open the blood-brain barrier in a very precise manner and document closure of the barrier within 24 hours," added study co-author Dr. Rashi Mehta, an associate professor at WVU. "The technique was reproduced successfully in the patients, with no adverse effects."
Even without drugs, opening of the brain-blood barrier in animals has shown positive effects, Mehta said. These effects may be due to increased flow of the fluid that cleans the brain of toxic substances, from an immune response triggered by the opening, or by some combination of the two.
While the research so far has focused on the technique's safety, the researchers plan to investigate MRgFUS therapeutic efficacy in the future.
"We'd like to treat more patients and study the long-term effects to see if there are improvements in memory and symptoms associated with Alzheimer's disease," Mehta concluded. "As safety is further clarified, the next step would be to use this approach to help deliver clinical drugs."