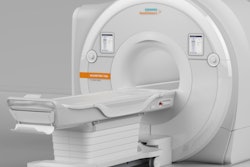
The U.S. Food and Drug Administration (FDA) has cleared Siemens Healthineers' Magnetom Sempra MRI system.
The 60-cm 1.5-tesla MRI system has low operating costs and a full-coverage service contract, the firm said. It also has Siemens' Day optimizing throughput (Dot) technology, which allows the user to adjust to each clinical case and the condition of each patient while simultaneously maintaining exam standardization.
 Magnetom Sempra from Siemens.
Magnetom Sempra from Siemens.Magnetom Sempra has three Dot engines for brain, spine, and large joint procedures, which together cover roughly three-quarters of the average MRI facility's exam volume.