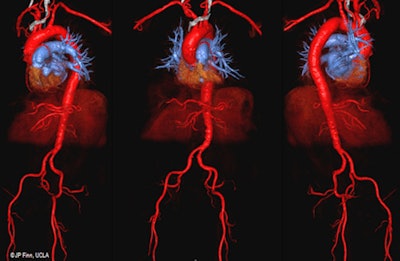
Given the concerns over the discovery of trace amounts of gadolinium in brain tissue long after contrast-enhanced MRI scans, researchers from the University of California, Los Angeles (UCLA) are reporting early success with an iron-based compound, ferumoxytol, to image a wide range of pediatric and adult patients.
The group performed a four-year retrospective study of more than 300 patients -- including those with at least stage 2 renal disease -- who couldn't undergo gadolinium-based MRI scans for fear of triggering a serious adverse event. While the study sample was small, the researchers are encouraged by ferumoxytol's safety record over several years at their institution.
 Dr. Kim-Lien Nguyen from UCLA.
Dr. Kim-Lien Nguyen from UCLA."Ferumoxytol allows us to image in a more individualized way. For certain patient populations, such as those with impaired renal function who are undergoing contrast-enhanced MRI exams, ferumoxytol offers another option," said lead author Dr. Kim-Lien Nguyen, an assistant professor of medicine at UCLA. "As we enter the age of patient-specific imaging, ferumoxytol allows us to choose the best MRI technique to provide value-added care."
Ferumoxytol's evolution
Dr. J. Paul Finn, senior investigator of and professor of radiological sciences at UCLA, initiated the clinical use of ferumoxytol-MRI at the request of nephrology colleagues. The group began their work shortly before reports began appearing in the literature of traces of gadolinium deposition in brain tissue long after administration of contrast agents. In late 2013, Kanda et al brought the issue to the forefront by discovering high signal intensities in the dentate nucleus and globus pallidus regions of patients who had undergone MRI scans with gadolinium-based contrast agents (GBCAs).
Since then, a series of follow-up studies have evaluated the gadolinium deposition issue in both adult and pediatric patient populations. In May, after a two-year review, the U.S. Food and Drug Administration (FDA) reported that it found no evidence that gadolinium remaining in the body after MRI contrast administration had any negative health effects. Still, the agency's declaration is unlikely to quell the debate over gadolinium contrast safety.
Gadolinium is a heavy metal that can be toxic to humans, so for use as an MRI contrast agent, it is linked, or chelated, into a compound that is designed to allow the agent's excretion. It's believed that for some agents these bonds can be broken, releasing free gadolinium into the body.
Ferumoxytol, on the other hand, is composed of small superparamagnetic iron oxide particles that can be detected with MRI. It is sold commercially as Feraheme by Amag Pharmaceuticals for the treatment of iron deficiency anemia in adult patients with chronic kidney disease.
 Peng Hu, PhD, from UCLA.
Peng Hu, PhD, from UCLA."Unlike many of the gadolinium-based contrast agents, ferumoxytol stays in the blood pool for a much longer time," said study co-author Peng Hu, PhD, an associate professor of radiological sciences at UCLA. "The half-life in the intravascular space is approximately 14 to 15 hours. That gives us a much wider time window to do more high-resolution and better-quality dynamic imaging."
While Feraheme's relative safety has been demonstrated for therapeutic use, it was the target of an FDA black box warning in 2015. The agency reported that "serious, potentially fatal allergic reactions can occur with the anemia drug Feraheme (ferumoxytol)" and added a "strong recommendation against use of Feraheme in patients who have had an allergic reaction to any intravenous iron replacement product."
A 2014 retrospective postmarketing therapeutic trial by Schiller et al of more than 8,600 patients at three U.S. dialysis centers evaluated ferumoxytol's safety record over a 12-month period. The researchers found a serious adverse event rate of 0.02% (range, 0.02%-1.3%) and "favorable assessments of long-term safety" (Clinical Therapeutics, January 2014, Vol. 36:1, pp. 70-83).
Still, Nguyen and colleagues noted in a March 2017 study that while "data on the safety of ferumoxytol as an off-label MRI contrast agent are accumulating steadily," information remains limited (Journal of Magnetic Resonance Imaging, March 2017, Vol. 45:3, pp. 804-812).
Adding to the need is the fact that ferumoxytol is the only contrast agent currently available for intravascular imaging. Lantheus Medical Imaging has discontinued the availability of its gadofosveset trisodium blood-pool GBCA (Ablavar) for suspected peripheral arterial occlusive disease. The company's manufacturing agreement for Ablavar has terminated, and there are no plans to initiate technology transfer activities for the product, according to Lantheus.
Study parameters
In the current study, presented at the International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting, the UCLA researchers reviewed 340 consecutive patients who underwent ferumoxytol MRI exams between 2013 and 2017. The subjects ranged in age from 3 days to 94 years and received a total of 372 injections of ferumoxytol, with approximately three dozen patients undergoing more than one scan.
Among the subjects, 242 (71%) had at least stage 2 chronic renal disease. The predominant indications for the ferumoxytol MRI exams scans were vascular (35%), congenital heart disease (27%), transplant (11%), transcatheter valve (8%), and cancer imaging (7%).
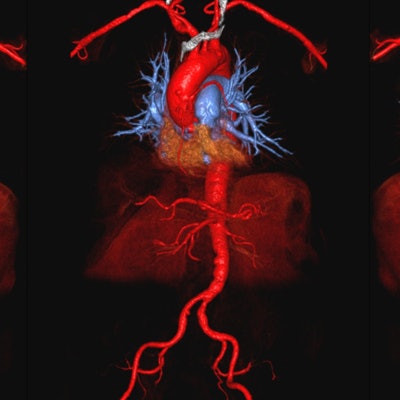
 3D color volume-rendered ferumoxytol-enhanced MR angiography images show excellent vascular mapping of the aortoiliofemoral arterial tree in an 87-year-old man with stage 4 renal disease, who is a candidate for transcatheter aortic valve replacement. Image courtesy of Nguyen et al.
3D color volume-rendered ferumoxytol-enhanced MR angiography images show excellent vascular mapping of the aortoiliofemoral arterial tree in an 87-year-old man with stage 4 renal disease, who is a candidate for transcatheter aortic valve replacement. Image courtesy of Nguyen et al.Nguyen and colleagues also took blood pressure, heart rate, oxygen saturation, and end-tidal CO2 readings from 23 ferumoxytol MRI patients under anesthesia and compared them with a comparable group of 23 patients who underwent MRI with gadofosveset under anesthesia.
While two patients developed mild cases of nausea related to the ferumoxytol MRI scans, there were no immediate adverse events, nor were there any severe, life-threatening, or fatal occurrences at follow-up (mean, 20 ± 12 months).
Likewise, no adverse events were reported in the ferumoxytol and gadofosveset subgroups. As for physiologic results, there was no statistically significant difference (p > 0.05) between the subgroups.
Promising results
The findings are promising for patients with chronic renal disease or renal impairment and adults who have diabetes, peripheral vascular disease, and other cardiovascular conditions such as heart failure.
"We no longer have to worry about renal function before performing contrast-enhanced MRI exams," Nguyen said. "And makes deposition of gadolinium a moot point. This is particularly important in very young children with congenital heart disease, because many will get repeated imaging over the course of their lifetime."
In fact, it has become routine for UCLA clinicians and surgeons to ask for ferumoxytol MRI scans, particularly for patients with vascular disease and anyone with impaired renal function. Patients with normal renal function have also undergone ferumoxytol MRI scans because of the quality of images obtained, Nguyen added.
UCLA researchers have also used ferumoxytol MRI for pediatric congenital heart disease patients and other vascular applications in adults. Small children, infants, and newborns need very high-resolution anatomical images of their hearts, Hu said, because they often have some form of complex congenital heart disease.
"The intravascular properties of ferumoxytol are very helpful in these cases, because it opens up the imaging window for a steady and stable [MR] signal," he said. "We do this on a regular basis now in very young children, with some as young as 2 days old."
Expanding applications
There is also interest in using ferumoxytol MRI for interventional planning because there is no radiation involved. The UCLA researchers have a study on this application pending publication.
In addition, they are exploring use of the contrast agent for different types of vascular disease within the abdomen and peripherally, while other researchers at the university are delving into ferumoxytol for inflammatory and cancer imaging.
"This agent is very versatile in terms of imaging characteristics," Hu said. "We are using [ferumoxytol] in T1-weighted MR imaging, which means that the blood pool is very bright. It also can be used as a T2 agent by using different types of pulse sequences. Because of the iron particles, it can be taken up by macrophages in the endothelial system or where there is inflammation. So it can be used as an inflammation contrast agent."
What will it take for more institutions to use ferumoxytol? Nguyen said acceptance starts with clinicians gaining experience in using it as a contrast agent.
"Ferumoxytol-enhanced has opened up conversations between radiologists and referring physicians, but more exchange is needed to identify which patient populations are best served with this agent," she said.
To that end, the researchers have been gathering data on their experiences with ferumoxytol to create a multicenter registry (FeraSafe) to share with other institutions. Nguyen said the initiative is "gaining traction," particularly with imaging centers that handle congenital heart disease and intravascular applications.
"We hope the registry will serve as a mechanism for centers to advance the diagnostic applications for ferumoxytol-enhanced MRI," she said.