Gadolinium retention in the brain may be more widespread than previous studies have shown, and it may not be limited to patients with brain abnormalities such as a tumor or infection, according to new research published online June 27 in Radiology.
The findings challenge previous theories regarding the permeability and role of the blood-brain barrier in the accumulation of gadolinium within the neural tissues. The blood-brain barrier, a highly selective semipermeable capillary network capable of selective filtration of blood products and molecules that cross into the brain tissues, was long thought to prevent agents like gadolinium-based contrast agents from entering extracellular fluid and cells within the central nervous system. Instead, the findings in the current study suggest that all adult patients could retain some level of gadolinium from a contrast-enhanced MRI scan.
"The reason why that is important is that there has always been this lingering thought that perhaps the presence of concomitant intracranial pathology, such as a tumors, rendered the blood-brain barrier -- even at distant sites -- more permeable," said lead author Dr. Robert J. McDonald, PhD, a staff neuroradiologist at the Mayo Clinic in Rochester, MN. "What we confirmed is that indeed these [gadolinium] agents do accumulate, even in the absence of intracranial pathology that could increase the permeability of the blood-brain barrier."
Accumulated research
For the past several years, there has been increasing evidence of gadolinium deposits accumulating in certain brain regions after multiple MRI scans using a gadolinium-based contrast agent (GBCA).
In a March 2015 study, McDonald and colleagues were the first to demonstrate convincing evidence of traces of gadolinium in the dentate nuclei, pons, globus pallidus, and thalamus in the autopsies of 13 deceased patients who received GBCAs between 2000 and 2014. The deposits appeared to occur in all patients exposed to gadolinium and were detectable with as few as four doses What's more, the patients had relatively normal renal function at the time of their MRI exams.
A few theories exist as to why gadolinium congregates in these brain areas. One hypothesis has to do with gadolinium's properties as a calcium analog.
"There is some thought that it is a case of mistaken identity whereby gadolinium is taken up in lieu of calcium at sites that ordinarily take up more calcium than other parts of the brain," McDonald told AuntMinnie.com. "These also happen to be neuroanatomic regions more prone to hemorrhage. It is possible that the reason why there is preferential accumulation is because the blood-brain barrier is less robust in these locations."
Whatever the reason, after a two-year review, the U.S. Food and Drug Administration (FDA) in May reported that it found no evidence that gadolinium remaining in the body after MRI contrast administration had any negative health effects. McDonald endorses the FDA's conclusions and feels more research is warranted to learn about the potential effects of GBCAs.
Therefore, he led a team of Mayo researchers who enrolled five patients (68 years; range, 47-73 years) in the GBCA-exposed group and 10 patients in a control group (79 years; range, 60-88 years). Deceased subjects in the GBCA-exposed group had undergone four to 18 separate contrast-enhanced MRI scans of the chest, abdomen, pelvis, and/or extremities between 2005 and 2015, compared with the control group whose subjects underwent at least one MRI brain scan but had no history of prior GBCA exposure.
The median age at the time of death was lower in the contrast group (68 years; range, 47-73 years) than in the control group (79 years; range, 60-88 years). The difference was not statistically significant (p = 0.27). The median time between the last MRI scan and death was, however, statistically and significantly shorter in the contrast group (56 days; range, 1-1,257 days) than the control group (727 days; range, 8-2,359 days) (p < 0.0001).
All patients underwent either a 1.5- or 3.0-tesla MRI scan (GE Healthcare or Siemens Healthineers) at the Mayo Clinic and received 0.1 mmol/kg of gadodiamide (Omniscan, GE Healthcare) as the GBCA for contrast-enhanced MRI. All subjects' median baseline renal function was normal at the time of the MRI scans, and postmortem evaluation of brain tissue found no underlying intracranial abnormalities.
Tissue samples from the subjects were harvested at the time of autopsyfrom the posterior fossa, which includes the dentate nucleus and pons, and supratentorial deep grey nuclei, which includes the globus pallidus and thalamus, and placed in formalin solution for further analysis.
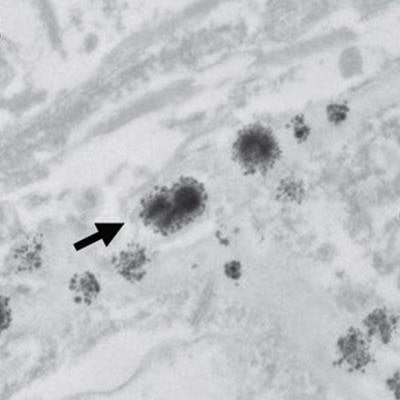
Researchers then performed inductively coupled plasma mass spectrometry and transmission electron microscopy with electron-dispersive x-ray spectroscopy to quantify the amount of gadolinium in brain tissues and characterize the distribution of gadolinium deposits in these formalin-fixed tissues.
Gadolinium levels
In reviewing the mass spectrometry data, the researchers detected gadolinium in the dentate, pons, globus pallidus, and thalamus of all five patients in the contrast group, with the highest concentrations ranging from 0.1 µg to 19.4 µg of gadolinium per gram of tissue (µg /g). There were no detectable levels of gadolinium among the 10 control subjects.
| Gadolinium distribution by brain regions | ||||
| Dentate | Pons | Globus pallidus | Thalamus | |
| Contrast group | ||||
| Patient 1 | 8.9 | 0.7 | 19.4 | 2.1 |
| Patient 2 | 6.7 | 0.2 | 9.0 | 1.1 |
| Patient 3 | 2.6 | 0.2 | 11.1 | 0.6 |
| Patient 4 | 2.2 | 0.7 | 2.1 | 0.9 |
| Patient 5 | 6.1 | 0.1 | 3.2 | 0.5 |
| All control subjects | 0.0 | 0.0 | 0.0 | 0.0 |
For each analyzed brain region, the cumulative gadolinium dose showed a moderate to strong correlation with tissue gadolinium concentration (dose to concentration ratio = 0.82-0.94, p < 0.04).
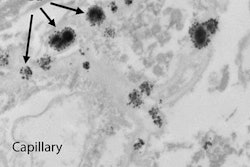
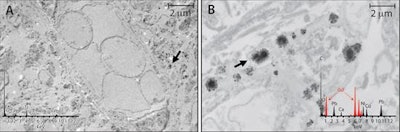 Tissue localization and cellular response to gadolinium deposition are displayed through transmission electron microscopy. Images show cellular localization of gadolinium in dentate nuclei tissue samples from a control patient (A) and from a patient who received gadodiamide (B). X-ray spectra are shown (inset) of each respective panel for selected electron-dense foci (arrows). Gadolinium peaks in spectra are indicated by red overlay. Images courtesy of Radiology.
Tissue localization and cellular response to gadolinium deposition are displayed through transmission electron microscopy. Images show cellular localization of gadolinium in dentate nuclei tissue samples from a control patient (A) and from a patient who received gadodiamide (B). X-ray spectra are shown (inset) of each respective panel for selected electron-dense foci (arrows). Gadolinium peaks in spectra are indicated by red overlay. Images courtesy of Radiology.Interestingly, the median gadolinium concentration in the globus pallidus increased from 1.5 µg/g in the 2015 study to 9.0 µg/g patients in the current study's contrast group; gadolinium concentrations in other brain regions remained about the same. While that may seem like a big increase, McDonald isn't sure it's significant.
"Although the deposition pattern differs somewhat from our prior study, the sample size is too small to draw any significant conclusions about this observation at this time," he added. "It would require a much larger study population to understand if there is a meaningful difference in the sites of preferential gadolinium deposition between patients with normal brain tissues and those with existing intracranial pathology."
Important facts
The Mayo researchers emphasized two important findings from the current study. First, the results may suggest that gadolinium accumulation may occur following every GBCA dose in the adult population.
"Second, it challenges our understanding of how and why this deposition is occurring," McDonald said. "These findings suggest our old model of the blood brain barrier and our assumptions that these deposits are directly traversing this barrier are both wrong. Our findings provide hints that gadolinium accumulation in tissues may occur through another, likely indirect, mechanism, that circumvents rather than directly crosses the blood brain barrier."
The authors cited several limitations of the study, including a small sample size and use of only a single linear GBCA (gadodiamide, limiting the generalizability of these findings..
Despite the limitations, the researchers are quick to note the importance of the findings, even in the small cohort, in understanding the scope of this issue.
"Currently, there is no convincing evidence from controlled scientific investigations that these intracranial deposits are toxic to the brain. Further it is important to understand that the amounts depositing within the brain are incredibly small and that extremely sensitive and expensive scientific and medical instrumentation is needed to detect these extremely low concentrations in the brain," McDonald said. "But these results underscore the importance of not just quantifying gadolinium deposition and, given the the scope of this issue, to undertake studies to better assess the safety of these deposits by studies aimed at evaluating their potential clinical manifestations."