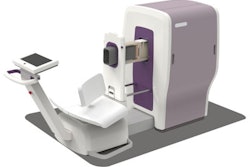
The U.S. Food and Drug Administration (FDA) has cleared Aspect Imaging's Embrace Neonatal MRI, the first such device for neonatal brain and head imaging in neonatal intensive care units, according to the agency.
Embrace Neonatal MRI can be used on newborns with a head circumference of up to 38 cm and who weigh between 2.2 lbs and 9.9 lbs. The system features a temperature-controlled incubator that minimizes movement of the baby. The baby can be removed from the system in less than 30 seconds, if needed, the FDA said.