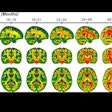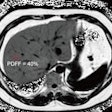
A four-year collaboration among GE Healthcare, the National Football League (NFL), and research institutions around the world culminated this week with U.S. Food and Drug Administration (FDA) 510(k) clearance for the vendor's Signa Premier MRI system.
The 3-tesla, wide-bore system was developed as part of a $60 million program launched by GE and the NFL in 2013 to develop imaging technologies that would advance the detection and diagnosis of concussions and speed appropriate treatment for players. The quest included $40 million to research, develop, and evaluate the next-generation modalities -- such as Signa Premier -- and $20 million was set aside to improve helmets and other protective devices.
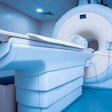
 The Signa Premier MRI system. Image courtesy of GE Healthcare.
The Signa Premier MRI system. Image courtesy of GE Healthcare.GE is highlighting the scanner's clinical and research capabilities -- particularly in the areas of neurology and oncology research. First showcased at RSNA 2016, Signa Premier features a new 70-cm bore, a 3-tesla short-bore superconductive magnet, and the SuperG gradient coil -- the most powerful gradient system GE has produced for a wide-bore, 3-tesla system. SuperG is designed to provide the performance of a research-class 60-cm MR system in a 70-cm bore, GE said.
Signa Premier also includes new, digital radiofrequency (RF) transmit and receive architecture. The scanner's RF technology offers 146 independent receiver channels, which simultaneously acquire patient data from multiple high channel-density surface coils. The result is faster scanning and enhanced image quality, according to the vendor.
The system also includes a 48-channel head coil, which features a fit-adaptable design that can accommodate 99.99% of the population, GE said. The company is also highlighting its HyperSense scanning tool, which can perform a routine fast brain examination in less than five minutes -- up to eight times faster than previous-generation GE MR scanners, according to the vendor. HyperSense is included in GE's HyperWorks application suite.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)