With the aid of MRI and machine learning, researchers have identified structural brain abnormalities that correspond with cognitive and behavioral deficiencies in people with common genetic causes of autism spectrum disorder, according to a new study published online August 8 in Radiology.
The key is a specific site on the 16th chromosome, known as 16p11.2. The deletion or duplication of a small piece of chromosome at this location is one of the most common genetic causes of autism spectrum disorder. People with this deletion tend to have brain overgrowth, developmental delays, and a higher risk of obesity, while individuals with duplications are born with smaller brains and tend to have lower body weight and developmental delays, the authors explained.
"By using unsupervised machine learning, we established a set of radiologic features that are emblematic of the deletion and duplication carriers," wrote the authors, led by Julia P. Owen, PhD, who was at the University of California, San Francisco (UCSF) during the study (Radiology, August 8, 2017). "Additionally, our results linking the radiology findings to the cognitive and behavioral scores could assist clinicians in assessing prognosis, although further study is warranted to fully establish these links."
With this information, researchers suggest MRI could play an influential role in identifying people with one of the most common genetic causes of autism and who are in most urgent need of intervention.
The researchers performed structural MRI scans from 2011 to 2015 at the University of Washington in Seattle, Baylor College of Medicine in Houston, and Harvard Medical School in Cambridge, MA.
The study included 79 carriers of a deletion at 16p11.2 (mean age 12.3 years; range 1-48 years) and 79 carriers of a duplication at 16p11.2 (mean age 24.8 years; range 1-63 years). To create a control group, researchers used 109 normal participants (mean age 25.5 years; range 6-64 years) and 64 unaffected family members (mean age 11.7 years; range 1-46 years). The structural 3D MR imaging protocol included T1-weighted, T2-weighted, and fluid-attenuated inversion-recovery (FLAIR) sequences. Participants also completed a series of cognitive and behavioral tests.
At least two of three board-certified neuroradiologists who were blinded to the genetic status of the patients independently reviewed the structural MR images for development-related abnormalities and used a checklist with 16 categories to identify congenital and developmental abnormalities for both the healthy controls and subjects with autism. A machine learning algorithm was used to "cluster radiologic features and an association between clusters and cognitive and behavioral scores from IQ tests" among the subjects, the authors noted.
MRI correlations
Their analysis showed some striking differences in the brain structures of carriers of deleted and duplicated chromosomes compared with noncarriers. For example, the corpus callosum was abnormally shaped and 16% thicker in the deletion carriers but 19% thinner in the duplication carriers, compared with the control group and family noncarriers.
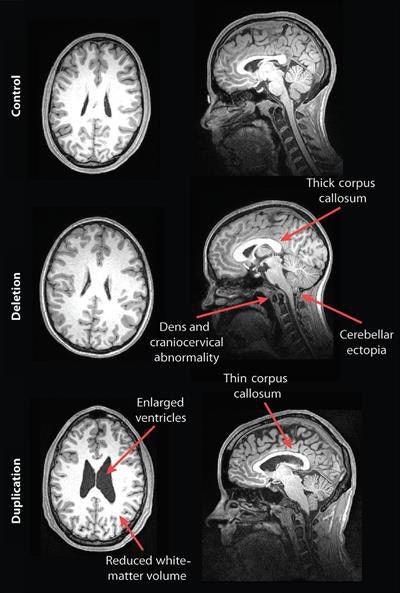
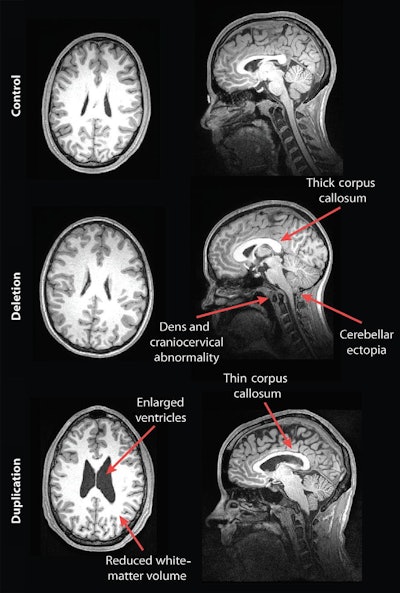
MR images of a control subject, deletion carrier, and duplication carrier. The deletion carrier's sagittal image shows a thick corpus callosum, dens and craniocervical abnormality, and cerebellar ectopia (arrows). The sagittal image for the duplication carrier shows the thin corpus callosum (arrow), while the axial image (left) displays increased ventricle size and decreased white-matter volume. Images courtesy of Radiology.
In addition, compared with unaffected family noncarriers and control participants, carriers with the deletion at 16p11.2 also exhibited signs of overgrowth in the posterior fossa, with a 31% likelihood of an abnormal downward displacement of the cerebellum, also known as cerebellar tonsillar ectopia (30.7%) and 9% greater occurrence of structural defects in the base of the skull and cerebellum.
Compared with unaffected family noncarriers and controls, carriers with the duplication at 16p11.2 showed signs of brain undergrowth, such as 23% decreased white-matter volume and 24% more ventricular volume.
As for the cognitive and behavioral results, the researchers were able to associate abnormal MRI findings among deletion carriers with worse daily living, communication, and social skills, compared with deletion carriers with no such abnormalities. In addition, the presence of decreased white matter and corpus callosal volume and increased ventricle size among the duplication carriers was associated with lower full-scale and verbal IQ scores, compared with duplication carriers without those findings.
"Often, studies like this focus on high-functioning individuals, but this was an 'all-comers' group," noted senior author Dr. Elliott Sherr, PhD, head of the Brain Development Research Program at UCSF, in a statement. "We didn't do mathematical algorithms but rather used the trained eyes of neuroradiologists to evaluate the scans of a full range of individuals. When you look at a broad range of people like this, from developmentally normal to more significantly challenged, you're better able