The U.S. Food and Drug Administration (FDA) has issued a letter to healthcare providers warning them that stainless steel neurovascular embolization coils could cause artifacts on MR angiography (MRA) images.
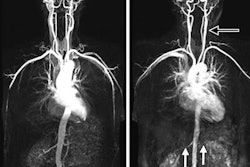
In a March 12 communiqué, the FDA advised users to check for 304V stainless steel in coils, which could cause "larger than expected artifact" in MRA images. The agency said it received information indicating that these coils could be causing artifacts that reduce image quality or result in inappropriate medical decisions.
Neurovascular embolization coils are used by physicians to treat brain aneurysms and other vascular abnormalities. Their purpose is to block blood flow and promote clotting in order to prevent a future hemorrhage.
Digital subtraction angiography (DSA) was previously used to evaluate the patient's condition and view the implanted coil, but healthcare providers have begun using MRA instead due to concerns over radiation from DSA.
If an artifact does occur, the agency recommends that clinicians use DSA to view aneurysms after coiling, rather than MRA. If MRA is the only option, the FDA advises using optimal imaging parameters to minimize any image artifact, including shortened echo times and high readout bandwidth.
Users are also encouraged to contact the coil manufacturer with any questions or concerns and to request specific recommendations about the use of the coil in MRA procedures, as well as to report any adverse events to the FDA.