Researchers found evidence of gadolinium accumulation in the brains of multiple sclerosis (MS) patients after MRI scans using a gadolinium-based contrast agent (GBCA), but the patients' conditions did not worsen by the five-year follow-up in a new study, published online July 5 in Neurology.
The retrospective, longitudinal study included only the linear GBCA gadodiamide (Omniscan, GE Healthcare) and included MS patients who were recently diagnosed with the condition to gauge how the GBCA might affect them with multiple MRI scans over the course of several years.
"The major strength of the present study is the selection of a homogenous patient population with 0.9 years of disease duration from onset at baseline that has been followed in our center from the time of diagnosis and has been assessed using the same GBCA with identical dose at all time points," wrote lead author Dr. Robert Zivadinov, PhD, and colleagues from the University at Buffalo's Jacobs School of Medicine and Biomedical Sciences.
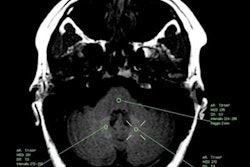
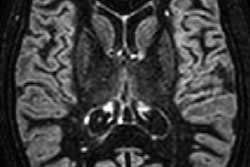
The results are particularly noteworthy since blood-brain barrier disruption can be a characteristic of MS and subsequently could allow gadolinium to accumulate in brain tissue of MS patients who undergo more GBCA-enhanced MRI scans because of their disease. One indication of gadolinium retention is believed to be increased signal intensity in the dentate nucleus on unenhanced T1-weighted MRI signals. Are MS patients at additional risk from repeated gadolinium contrast-enhanced MRI scans, then?
To explore this question, the researchers compared 203 MS patients who underwent gadodiamide-enhanced MRI scans on the same 3-tesla scanner (Signa Excite HD, GE Healthcare) between 2003 and 2016 against 262 age- and sex-matched healthy controls. At baseline, MS patients had the disease for less than two years and were scanned only with gadodiamide at all MRI time points. Over the course of the mean follow-up time of 55.4 months, MS patients had received a mean of 9.2 gadodiamide administrations. To gauge gadolinium accumulation in the brain, the researchers then calculated signal intensity ratios of the dentate nucleus, globus pallidus, and thalamus to the pons of all subjects.
They found statistically significant differences in signal intensity ratios for the dentate nucleus and global pallidus with the pons among MS patients, compared with healthy controls. Interestingly, the thalamus-to-pons signal intensity ratio was significantly higher for the healthy controls than for the MS patients.
"The lower thalamic ratio in patients with MS may potentially reflect decreased iron content compared to controls in this structure, as has been recently reported using quantitative susceptibility mapping," the researchers noted.
| Signal intensity ratios between MS patients and healthy controls | |||
| MS patients | Healthy controls | p-value* | |
| Dentate nucleus-to-pons | 1.056 | 1.038 | < 0.001 |
| Global pallidus-to-pons | 1.047 | 1.028 | < 0.005 |
| Thalamus-to-pons | 0.951 | 0.977 | < 0.001 |
The number of GBCA doses also was a factor in the degree of signal intensity. The dentate nucleus-to-pons ratio was significantly greater among the 109 MS patients with eight or more gadodiamide doses (1.064), compared with the 94 MS patients with fewer than eight doses (1.045; p = 0.005).
While the researchers noted increased signal intensity from the GBCA, they did not see evidence of progression to more severe disease over the follow-up among any MS patients. More specifically, they noted no significant differences in the number of relapses among MS patients, no brain atrophy, and no indications that gadolinium contributed to any increased disability among the patients.
The group did note, however, one caveat to the findings. While the team found no discernible clinical outcome, patients who received more than eight GBCA doses had more brain lesions and more advanced atrophy of gray matter, compared with MS patients who had fewer than eight doses. Therefore, Zivadinov and colleagues could not definitively rule out that gadolinium deposition may have an adverse effect on disease progression or clinical outcome.