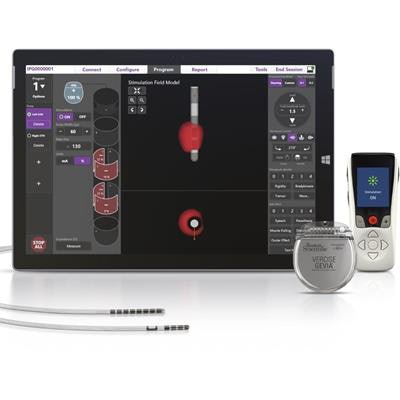
The U.S. Food and Drug Administration (FDA) has approved the use of Boston Scientific's Vercise Gevia deep brain stimulation (DBS) system during full-body MRI exams.
Vercise Gevia, in conjunction with the Vercise Cartesia directional lead implantable pulse generator, is designed to deliver targeted electrical stimulation to the brain for patients with Parkinson's disease. The FDA approval allows clinicians to monitor patients undergoing deep brain stimulation therapy using MRI.
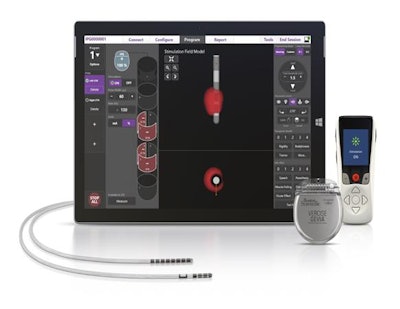 The Vercise Gevia deep brain stimulation system. Image courtesy of Boston Scientific.
The Vercise Gevia deep brain stimulation system. Image courtesy of Boston Scientific.The Vercise Gevia DBS system has been commercially available in Europe since 2017.