GE Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for AIR Recon DL, an artificial intelligence technology that improves MR image quality.
The reconstruction technology runs on GE's Edison deep-learning platform and helps boost image resolution to allow for shorter MRI scan times. The algorithm can improve the signal-to-noise ratio and suppress ringing of scans, the company noted.
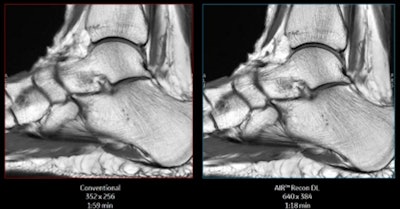 GE's AIR Recon DL delivers shorter scans and better image quality. Image courtesy of GE Healthcare.
GE's AIR Recon DL delivers shorter scans and better image quality. Image courtesy of GE Healthcare.Hospitals from around the world helped to develop AIR Recon DL, which has been tested on thousands of cases, according to GE. The technology is available for the company's 3-tesla MR systems as an upgrade or with a new purchase.