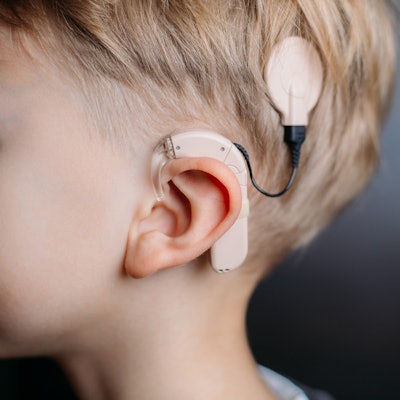
Several hundred adverse events during MRI scans of patients with implantable hearing devices have occurred in the past decade, particularly in patients with cochlear implants, Belgian researchers are reporting at this week's International Society for Magnetic Resonance in Medicine (ISMRM) virtual conference.
"Patients suffering from disabling hearing loss are often treated via the implantation of an active auditory implant," Guy Fierens, a research engineer at the Cochlear Technology Center in Mechelen, and his colleagues explained in a digital poster at ISMRM 2020. "The use of MRI is often needed in a postoperative follow-up or for the diagnosis of other pathologies. Using MRI in this patient group, however, raises some concern due to several possible patient risks."
The goal of the authors was to describe the most commonly occurring adverse events by analyzing data reported to the U.S. Food and Drug Administration (FDA), which are based largely on the Manufacturer and User Facility Device Experience (MAUDE) database. The researchers also conducted a search of the Medline and Embase literature.
| Adverse events during MRI scans of patients with implantable hearing devices over 10 years | |
| Total adverse events | 624 |
| Cochlear implants | 592 |
| Bone conduction implants | 15 |
| Middle ear implants | 13 |
| Auditory brainstem implants | 2 |
In 130 of these events, information was given about manufacturer guidelines. The guidelines were followed in 61 cases (47%), and the guidelines were not adhered to in 49 cases (37%). In the remaining cases, it was unclear whether the guidelines had been followed.
The most common cause of adverse events was dislocation of magnets in implantable devices during MRI exams. This occurred in 384 cases, of which 59 were painful.
"Most implant manufacturers offer the opportunity of removing the magnet before an MRI exam, but this also carries some risks," noted Fierens, who is also a researcher at KU Leuven and an employee of Cochlear Limited.
A total of 18 adverse events were reported due to the removal of the magnet prior to MRI. Of these, 11 were tissue reactions, three were tears in silicone, and four were explant procedures labeled as adverse events.
In 110 cases, the entire implant was removed prior to MRI at the request of the patient or because it was the preference of the clinician. In another 48 cases, the patients described the procedure as being painful, mostly in terms of the surrounding tissue. Malfunctioning of the implanted device was also a rare cause of adverse events.
Overall, the patients complained of pain in 88 cases (17%). Of these cases, 50 patients wore a headband, which is often recommended, and one-third were unable to complete the scan. Dislocation of the implantable device's magnet occurred in 58 cases (11%), while image artifacts obstructing the region-of-interest were reported in 52 cases.
"Our study shows that manufacturer guidelines are often not followed," he concluded. "The most common adverse events were dislocation of the implanted magnet and pain from overlying tissue, often leading to a premature ending of the procedure. Dislocation is possible even with the recommended splint or headband."