The U.S. Food and Drug Administration (FDA) cleared two new artificial intelligence (AI)-based MRI interpretation applications from Siemens Healthineers.
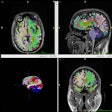
The company's AI-Rad Companion Brain MR for Morphometry Analysis is an algorithm that automatically measures volumetric changes in the brain. Brain volumetry involves measuring the volume of gray matter, white matter, and cerebrospinal fluid in the brain, and it previously was performed manually or semiautomatically. Lower volume can indicate the presence of Parkinson's or Alzheimer's disease.
Meanwhile, AI-Rad Companion Prostate MR for Biopsy Support segments the prostate on MR images and marks the organ's outer contour in seconds, much faster than manual segmentation.
Radiologists can then mark suspicious regions and send annotated MR images to urologists for fusion with ultrasound images during biopsy procedures.
The software aims to give radiologists more time to focus on the most important tasks during MRI exams.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)