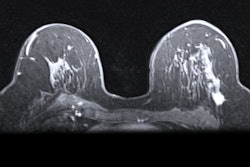
The COVID-19 vaccine appears to increase incidence of axillary lymphadenopathy on breast MRI -- thus boosting women's risk of unnecessary biopsy, according to a study published February 5 in the American Journal of Roentgenology.
These initial clinical experiences suggest that an additional protocol may be needed for breast MRI in the COVID-19 era, wrote a team led by Dr. Christine Edmonds of the Hospital of the University of Pennsylvania in Philadelphia.
"Early clinical experience with coronavirus disease (COVID-19) vaccination suggests that the approved COVID-19 vaccines cause a notably higher incidence of axillary lymphadenopathy on breast MRI compared to other vaccines," the group wrote. "Guidelines are needed to appropriately manage MRI-detected unilateral axillary lymphadenopathy in the era of COVID-19 vaccination and to avoid biopsies of benign reactive nodes."
Edmonds' team examined available clinical data on vaccine-related lymphadenopathy and shared a strategy to assess it and guide breast MRI management. The strategy includes adding questions to breast imaging intake forms regarding dates of COVID-19 vaccination, as well as where the vaccine was administered.
"MRI-detected isolated unilateral axillary lymphadenopathy ipsilateral to the vaccination arm to be most likely COVID-19 vaccine-related if within four weeks of either dose," the team wrote.
If lymphadenopathy is discovered in patients who have received the COVID-19 vaccine, the team suggests categorizing it as BI-RADS 3 and recommending a follow-up ultrasound six to eight weeks after the second vaccine dose.
"These guidelines may be refined as we gain further data on the expected time-course of axillary lymphadenopathy post COVID-19 vaccination," Edmonds and colleagues concluded. "Until that time, this management pathway will help avoid unnecessary biopsies of benign vaccine-related reactive lymphadenopathy."