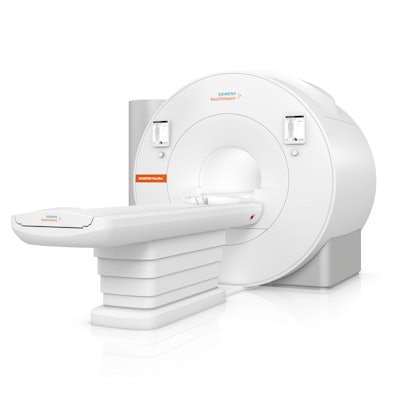
Siemens Healthineers has received clearance from the U.S. Food and Drug Administration (FDA) for Magnetom Free.Star, a compact MRI scanner with a 60-cm magnet bore.
The new system is a more compact, lighter version of Magnetom Free.Max, a scanner with an 80-cm bore that was first launched in 2021. Both scanners are superconducting 0.55-tesla magnets in the company's High-V MR product line and require less than 1 liter of liquid helium and no quench pipe, making siting and operation easier, according to the company.
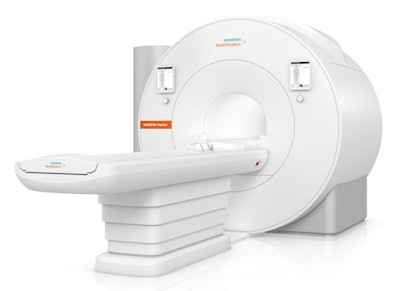 Magnetom Free.Max becomes Siemens' lightest and most compact whole-body MRI scanner.
Magnetom Free.Max becomes Siemens' lightest and most compact whole-body MRI scanner.Free.Star becomes the most affordable and lightweight whole-body MRI scanner in the company's product line, weighing in at 3.3 tons versus 3.5 tons for Free.Max. Its operating costs are 30% lower than a conventional MRI scanner, according to Siemens.
The scanner also includes Siemens technologies like Deep Resolve algorithms for denoising to produce sharper, high-resolution images, and myExam Companion to enhance workflow for conducting more efficient patient exams.



















