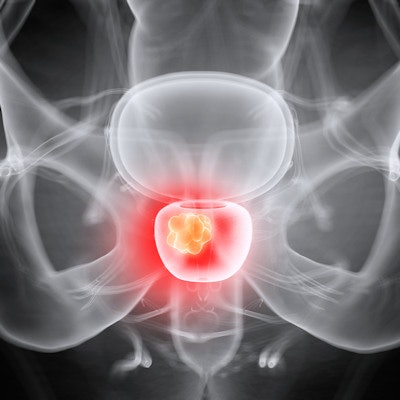
MRI software developer Bot Image's ProstatID artificial intelligence (AI) software has received U.S. Food and Drug Administration (FDA) clearance for detection and diagnosis of prostate cancer on MRI.
After recognizing and measuring prostate gland volume and detecting suspicious cancerous lesions, the software estimates a cancer probability for these lesions and suggests a Prostate Imaging Reporting and Data System (PI-RADS) diagnostic score for each case, according to the vendor.
Bot Image is making ProstatID available as a software-as-a-service, requiring a virtual private network (VPN) connection between the cloud-based ProstatID server and the radiology department server or MRI system.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











