Sunday, November 27 | 2:30 p.m.-3:30 p.m. | S5-SSMS01-6 | Room E351
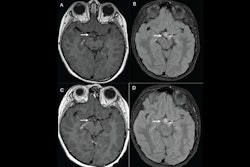
An investigational macrocyclic gadolinium-based contrast agent shows promise for use for contrast-enhanced MRI -- at half the dose of other agents, according to research to be presented during this Sunday scientific session.Presenter Dr. Christiane Kuhl, PhD, of University Hospital Aachen in Germany will share results from the PROMISE trial, which compared the use of gadopiclenol at 0.05 mmol/kg to gadobutrol at 0.1 mmol/kg for body MRI. The research included 304 patients with lesions across a variety of areas who underwent two MRIs using both of the agents. Three readers assessed the performance of the agents according to the following measures: border delineation, internal morphology, and degree of contrast enhancement. Kuhl and colleagues calculated percentage of enhancement and lesion-to-background ratio for each agent and tracked any adverse events patients may have experienced.
The results? The two agents were comparable when it came to visualizing lesions, and there were no significant differences between the two for lesion-to-background ratio. The three readers reported no preference for either agent in 74.6% to 82.6% of the exams, and the incidence of adverse events (mostly injection site reactions) was comparable between the two, at 18.1% for gadopiclenol and 20% for gadobutrol.
The research suggests that gadopiclenol could be an effective, lower-dose alternative to gadobutrol -- which could address concerns about gadolinium retention and its potential negative effects. Stop by this presentation on Sunday to get more information.